Topic 8 Acids and alkalis
advertisement
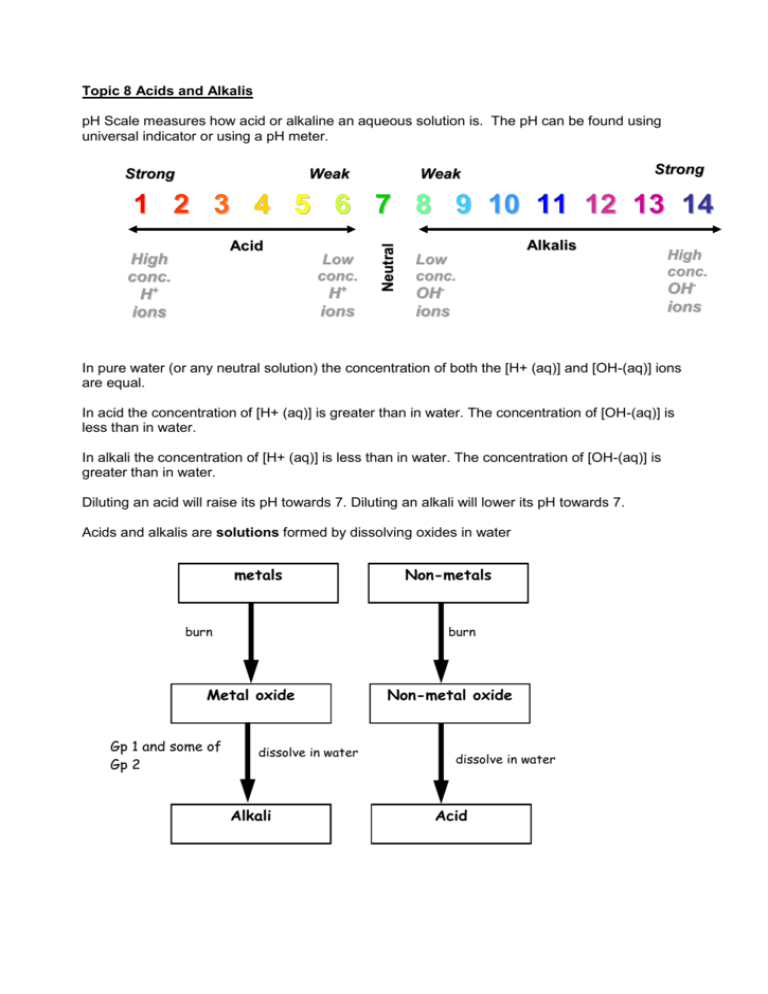
Topic 8 Acids and Alkalis pH Scale measures how acid or alkaline an aqueous solution is. The pH can be found using universal indicator or using a pH meter. Strong Weak Strong Weak Acid s High conc. H+ ions Low conc. H+ ions Neutral 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Alkalis Low conc. OHions High conc. OHions In pure water (or any neutral solution) the concentration of both the [H+ (aq)] and [OH-(aq)] ions are equal. In acid the concentration of [H+ (aq)] is greater than in water. The concentration of [OH-(aq)] is less than in water. In alkali the concentration of [H+ (aq)] is less than in water. The concentration of [OH-(aq)] is greater than in water. Diluting an acid will raise its pH towards 7. Diluting an alkali will lower its pH towards 7. Acids and alkalis are solutions formed by dissolving oxides in water metals burn burn Metal oxide Gp 1 and some of Gp 2 Non-metals dissolve in water Alkali Non-metal oxide dissolve in water Acid Acid Rain History Acid rain is a widespread term used to describe all forms of acid precipitation (rain, snow, hail, fog, etc.) which when tested with indicator solution has a pH less than 7. The term acid rain was first used by Robert Angus Smith, a scientist working in Manchester in the 1870s. The problem of acid rain is hence not a new one but the nature of the problem has changed from being a local problem for towns and cities to being an international problem. In Smith’s time, acid rain fell both in towns and cities whilst today pollutants can be transported thousands of kilometres due to the introduction of tall chimneys dispersing pollutants high into the atmosphere. Causes Precipitation is naturally acidic because of carbon dioxide in the atmosphere. The burning of fossil fuels (coal, oil and gas) produces sulphur dioxide and carbon dioxide and the sparking of air in car engines produces nitrogen oxides which can increase the acidity of rain or other precipitation when converted to sulphuric, carbonic and nitric acids. This acidic pollution can be transported by wind over many hundreds of miles, and deposited as acid rain. Every year, the use of energy in buildings requires the burning of large quantities of fossil fuels. This produces millions of tones of gases such as sulphur dioxide and carbon dioxide which are released into the atmosphere. Nitrogen Oxides Nitrogen oxides (also known as oxides if nitrogen, and abbreviated as NOx) is a collective term used to refer to two species of oxides of nitrogen: nitric oxide (NO) and nitrogen dioxide (NO2). Nitrogen dioxide is a strong oxidizing agent that reacts in the air to form corrosive nitric acid. Sulphur Dioxide Sulphur dioxide (SO2) is a colourless gas, belonging to the family of gases called sulphur oxides (SOx). It is soluble in water and in the atmosphere, sulphur dioxide dissolves in water vapour producing sulphuric acid. . Sources of sulphur dioxide,oxides of nitrogen and carbon dioxide may be natural such as volcanoes, oceans, biological decay and forest fires, or may arise from combustion sources. The increasing demand for electricity and the rise in the number of motor vehicles in recent decades has meant that emissions of acidifying pollutants have increased dramatically from human sources, particularly since the 1950s. The most important man-made sources of sulphur dioxide are fossil fuel combustion, smelting, manufacture of sulphuric acid, conversion of wood pulp to paper, incineration of refuse and production of elemental sulphur. Coal burning is the single largest man-made source of sulphur dioxide. Locations In the 1970s and 1980s, Scandinavian countries began to notice the effects of acid deposition on trees and freshwaters. Much of the pollution causing this damage was identified as being transported from other more polluting countries. Acid rain turned into an international concern and became particularly prominent as a media issue during the 1980s. However, during the 1970s many countries started to notice changes in fish populations in lakes and damage to certain trees. By the late 1970s concern led to international efforts to identify the causes and effects of long-range (transboundary) transport of air pollutants, and thus during the 1980s much research was conducted in Europe and North America. Effect on Materials Buildings have always been subject to attack by weathering; the effects of rain, wind, sun, and frost. Acid rain can accelerate the rate of this damage. It affects most materials to some degree. Limestone, marble and sandstone are particularly vulnerable. Limestone and marble turn to a crumbling substance called gypsum upon contact with the acid, which explains the corrosion of buildings and statues. Other vulnerable materials include carbon-steel, nickel, zinc, copper, paint, some plastics, paper, leather and textiles. Structural damage to underground pipes, cables and foundations submerged in acid waters can also occur, in addition to damage to buildings, bridges and vehicles above ground. In addition, bridges are corroding at a faster rate, and the railway industry as well as the airplane industry have to expend more money in repairing the corrosive damage done by acid rain. Not only is this an economically taxing problem caused by acid rain, but also a safety hazard to the general public. Effect on Aquatic Life Thousands of lakes and rivers there have become acidified. Acid sensitive species are absent from around 40% of Sweden’s rivers and streams. Sulphuric acid (H2SO4) directly interferes with the fish's ability to take in oxygen, salt and nutrients needed to stay alive and increased death rates in fish are noticed. This in turn leads to an increased rate of natural decomposition of dead fish and the decomposition process in turn uses up a lot of the oxygen, which leaves less for the surviving fish to take in. Amphibians are also affected; like the fish, they cannot reproduce in an acidic environment. The amphibian embyos have membranes that are too tough because of the acids, such that they are unable to break through at the proper time. So, they continue to grow, only to have deformed spines. They are then killed by a fungus that has been allowed to grow on their membranes. Hence, in essence, the effects of acid rain on lakes and its aquatic ecosystem are numerous and over-whelmingly magnified as we move down the food web. Effect on Trees Acid rain can have serious impacts on trees and forests. Acid rain does not usually kill trees directly. Instead, it is more likely to weaken them by damaging their leaves, limiting the nutrients available to them, or poisoning them with toxic substances slowly released from the soil. Thus, the trees are starved to death as they are deprived of their vital nutrients such as calcium and magnesium. Nutrients present in the soils are washed away. Aluminium also present in the soil is freed and this toxic element can be absorbed by the roots of trees. Effect on Humans An indirect effect of acid precipitation on humans is that the toxic metals dissolved in the water are absorbed in fruits, vegetables and in the tissues of animals. Although these toxic metals do not directly affect the animals, they have serious effects on humans when they are being consumed. Fish, being one of the primary members of the food chain, is food for many other life-forms, including humans. Because toxic materials such as mercury are deposited in the fish due to acid rain, it is dangerous for humans to consume the fish as it has been linked with brain damage in children as well as nerve disorders, brain damage and death. Like the domino effect, fewer fish can be sold as food, fishermen lose their hobby and people selling fishing supplies are affected. Similarly, another metal, Aluminium, present in the organs of the animals, has been associated with kidney problems and recently, was suspected to be related to Alzheimer's disease. Actions International legislation during the 1980s and 1990s has led to reductions in sulphur dioxide emissions in many countries but reductions in emissions of nitrogen oxides have been much less. The UK is committed to reducing sulphur emissions through introducing stricter regulations. These require UK to reduce sulphur emissions by 50% by year 2000, 70% by 2005 and 80% by 2010 (all on 1980 levels). To meet these requirements, emissions of sulphur dioxide in UK are being reduced, through the use of cleaner technology within the power generation industry.









