Atom inventory * balancing equations
advertisement

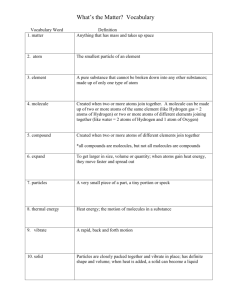
WEDNESDAY, OCTOBER ST 21 Please do not touch the materials on your desks Take out your notebook and a writing utensil and copy down the following: The law of conservation of mass tells us that matter is never created nor destroyed. The number of atoms at the beginning of a chemical reaction must equal the number of atoms at the end You can count the atoms present in a chemical reaction to ensure that the amounts match BALANCING EQUATIONS Purpose of today: To understand how the “recipe” for reactions is created To practice balancing chemical equations CHEM CATALYST Does this equation obey the law of conservation of mass? Why or why not? CuCl2 (aq) + Na2S (aq) → CuS(s) + NaCl (aq) WRITING CHEMICAL EQUATIONS Kind of like a mathematical formula X + Y → XY XY → X + Y Stuff to the left of the arrow: reactants Stuff to the right of the arrow: products Reactants react to form products! SETUP Work with your table groups You should have One set of worksheets 1 “Block Molecules” sheet 1 “Equation 1” sheet 1 “Equation 3” sheet Dry erase marker and rag Beaker of colored blocks Your notebook and a pen/pencil TASK 1 Consider the following chemical equation: Zn (s) + HCl (aq) → ZnCl2 (aq) + H2 (g) This equation tells us that solid zinc atoms react with hydrochloric acid molecules to produce zinc (II) chloride molecules dissolved in water and hydrogen gas. Using the colored blocks, create a surplus (lots) of each of the reactants and the products Zinc = purple Hydrogen = yellow Chloride = blue TASK 2 Display the # of atoms/molecules, according to the chemical equation, on the “Block Molecules” worksheet Using the “Atom Inventory Chart” on the Reaction 1 worksheet, count the atoms of each type in the reactants and the products Fill in the chart using the dry erase marker Does the reaction follow the Law of Conservation of Mass (matter)? Is the reaction balanced? If not, add more atoms or molecules to the “Block Molecules” display worksheet accordingly Change the numbers in your inventory chart to reflect the changes you made by adding atoms/molecules to the reactants and/or products TASK 3 Use coefficients (large numbers written in front of molecular formulas) to show how many of each reactant is need and how many of each product is made __ Zn (s) + __ HCl (aq) → __ZnCl2 (aq) + __ H2 (g) Coefficients go here! BALANCE THE REMAINING EQUATIONS Steps to follow using the blocks: 1. Create a surplus of the reactants and products 2. Organize the correct # of atoms/molecules onto the “Block Molecules” display sheet (according to the chemical equation) 3. Fill in an atom inventory chart to determine if the reaction is balanced – If not, add more atoms/molecules accordingly to the Block Molecules worksheet to balance 4. Change the numbers on your inventory chart to reflect addition of reactants or products 5. Fill in the coefficients to reflect number of reactant atoms/molecules needed and number of product atoms/molecules made 6. Get your work checked by me after each equation!! CLEAN-UP Separate ALL blocks and put back in beakers Erase worksheets Leave everything as you found it Write the following reaction in your notebook __ Ca (s) + __ O2 (g) → __ CaO (s) Create an atom inventory chart Balance the reaction BALANCING WITHOUT BLOCKS! Write the following reaction in your notebook __ Ca (s) + __ O2 (g) → __ CaO (s) Create an atom inventory chart Balance the reaction ATOM INVENTORY EXIT SLIP Complete on your own and turn in before you leave!!