Chapter 11: Acid-Base Titrations
advertisement
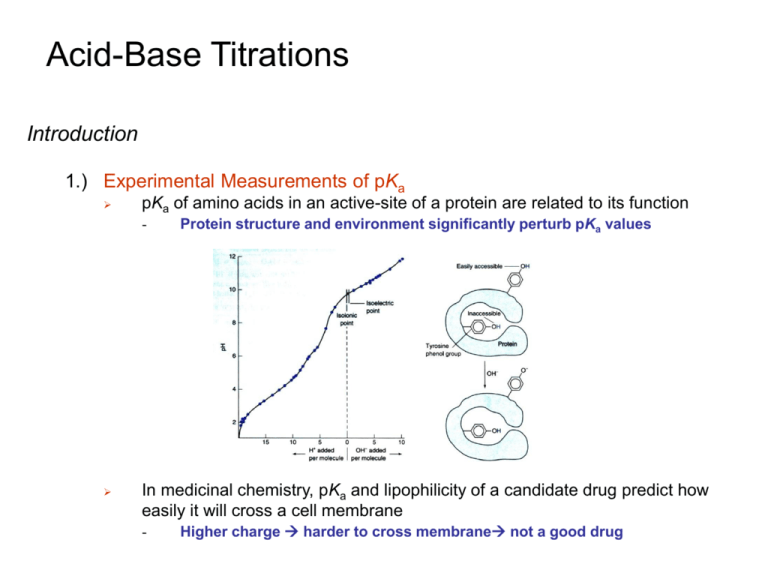
Acid-Base Titrations Introduction 1.) Experimental Measurements of pKa pKa of amino acids in an active-site of a protein are related to its function - Protein structure and environment significantly perturb pKa values In medicinal chemistry, pKa and lipophilicity of a candidate drug predict how easily it will cross a cell membrane - Higher charge harder to cross membrane not a good drug Acid-Base Titrations Introduction 2.) Example: impact of the Asp on the pKa of His in the His-Asp catalytic dyad. - Glucose 6-phosphate dehydrogenase (G6PD) catalyzes the oxidation of glucose 6-phosphate using NAD+ or NADP+ His-240 is the general base that extracts a proton from the C1-OH of G6P The pKa of His-240 in the G6PD apoenzyme is found to be 6.4, which corresponds to an unidentified pKa value of 6.3 that was previously derived from the dependence of kcat on pH. These results suggest that the pKa of His-240 is unperturbed by Asp. Biochemistry, Vol. 41, No. 22, 2002 6945 Acid-Base Titrations Introduction 3.) Overview Titrations are Important tools in providing quantitative and qualitative data for a sample. To best understand titrations and the information they provide, it is necessary to understand what gives rise to the shape of a typical titration curve. To do this, acid-base equilibria are used to predict titration curve shapes. proton release from PAA decreases with increase in the degree of dissociation for the highest polymer concentration conformational change of the PAA from rodlike conformation to a random coil form, J. Phys. Org. Chem. 2006; 19: 129–135 Acid-Base Titrations Titration of Strong Base with Strong Acid 1.) Graph of How pH changes as Titrant is Added Assume strong acid and base completely dissociate Any amount of H+ added will consume a stoichiometric amount of OHK 1 Kw 10 14 Reaction Assumed to go to completion Three regions of the titration curve - Before the equivalence point, the pH is determined by excess OH- in the solution - At the equivalence point, H+ is just sufficient to react with all OH- to make H2O - After the equivalence point, pH is determined by excess H+ in the solution. Acid-Base Titrations Titration of Strong Base with Strong Acid 1.) Graph of How pH changes as Titrant is Added Remember, equivalence point is the ideal goal Actually measure End Point - Different Regions require different kinds of calculations - Marked by a sudden physical change: color, potential Illustrated examples The “true” titration reaction is: Titrant Analyte Acid-Base Titrations Titration of Strong Base with Strong Acid 2.) Volume Needed to Reach the Equivalence Point Titration curve for 50.00 mL of 0.02000 M KOH with 0.1000 M HBr At equivalence point, amount of H+ added will equal initial amount of OH- Ve ( mL )0.1000 M 50.00 mL ( 0.02000 M ) Ve 10.00 mL mmol of HBr at equivalence point mmol of OHbeing titrated When 10.00 mL of HBr has been added, the titration is complete. Prior to this point, there is excess OH- present. After this point there is excess H+ present. Acid-Base Titrations Titration of Strong Base with Strong Acid 3.) Before the Equivalence Point Titration curve for 50.00 mL of 0.02000 M KOH with 0.1000 M HBr Equivalence point (Ve) when 10.00 mL of HBr has been added When 3.00 mL of HBr has been added, reaction is 3/10 complete - Initial volume of OHCalculate Remaining [OH-]: 50.00 10.00 3.00 [OH ] 0.02000 M 0.0132 M 10.00 50.00 3.00 Fraction of OH Initial concentration Dilution Factor Remaining of OH Total volume Calculate [H+] and pH: 1.0 10 14 13 [H ] 7 . 58 10 M pH 12.12 0 . 0132 [OH ] Kw Acid-Base Titrations Titration of Strong Base with Strong Acid 4.) At the Equivalence Point Titration curve for 50.00 mL of 0.02000 M KOH with 0.1000 M HBr - Just enough H+ has been added to consume OHpH determined by dissociation of water Kw x x Kw= 1x10-14 Kw x 2 x 1.00 10 7 M pH 7.00 - pH at the equivalence point for any strong acid with strong base is 7.00 Not true for weak acid-base titration Acid-Base Titrations Titration of Strong Base with Strong Acid 5.) After the Equivalence Point Titration curve for 50.00 mL of 0.02000 M KOH with 0.1000 M HBr - Adding excess HBr solution When 10.50 mL of HBr is added Calculate volume of excess H+: Vadded Vequivalence 10.50 10.00 0.50 mL Calculate excess [H+]: Volume of excess H+ 0.50 4 [H ] 0.1000 M 8.26 10 M 50.00 10.50 Initial Total volume concentration Dilution factor of H+ Calculate pH: pH -log[ H ] log( 8.26 10 4 M ) 3.08 Acid-Base Titrations Titration of Strong Base with Strong Acid 6.) Titration Curve Rapid Change in pH Near Equivalence Point - Equivalence point is where slope is greatest Second derivative is 0 pH at equivalence point is 7.00, only for strong acid-base - Not True if a weak base-acid is used Acid-Base Titrations Titration of Weak Acid with Strong Base 1.) Four Regions to Titration Curve Before any added base, just weak acid (HA) in water - pH determined by Ka Ka With addition of strong base buffer - pH determined by Henderson Hasselbach equation [ A ] pH pK a log [HA] At equivalence point, all HA is converted into A- Weak base with pH determined by Kb Kb Acid-Base Titrations Titration of Weak Acid with Strong Base 1.) Four Regions to Titration Curve Beyond equivalence point, excess strong base is added to A- solution - pH is determined by strong base Similar to titration of strong acid with strong base 2.) Illustrated Example: Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH - MES is a weak acid with pKa = 6.27 K - Reaction goes to completion with addition of strong base K 1 7 1 1 5 . 4 10 Kb Kw / K a 1 10 14 / 10 6.27 Acid-Base Titrations Titration of Weak Acid with Strong Base 3.) Volume Needed to Reach the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH - Reaction goes to completion with addition of strong base Strong plus weak react completely Ve ( mL )0.1000 M 50.00 mL ( 0.02000 M ) Ve 10.00 mL mmol of base mmol of HA Acid-Base Titrations Titration of Weak Acid with Strong Base 4.) Region 1: Before Base is Added Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Simply a weak-acid problem Ka Calculate [H+]: F-x Ka= 10-6.27 x x x2 x2 K a x [H ] 1.03 10 4 F x 0.02000 x Calculate pH: pH - log [H ] log( 1.03 10 4 M ) 3.99 Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Region 2: Before the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Adding OH- creates a mixture of HA and A- Buffer Calculate pH from [A-]/[HA] using Henderson-Hasselbach equation Simply the difference Simply of volumes of initialratio quantities Calculate [A-]/HA]: Amount of added NaOH is 3 mL with equivalence point is 10 mL Relative Initial quantities (HA≡1) 1 3 10 - Relative Final quantities 7 10 - 3 10 Calculate pH: 3 [ A ] 6.27 log 10 5.90 pH pK a log 7 [HA] 10 3 10 Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Region 2: Before the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH pH = pKa when the volume of titrant equals ½Ve Relative Initial quantities (HA≡1) Relative Final quantities 1 1 2 1 2 - - 1 2 1 [ A ] pK log 2 pK pH pK a log a a 1 [HA] 2 1 2 Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Region 3: At the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Exactly enough NaOH to consume HA Relative Initial quantities (HA≡1) 1 1 - - Relative Final quantities - - 1 1 The solution only contains A- weak base Kb F-x Kb x K x2 Kb w Fx Ka x Kw Ka Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Region 3: At the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Calculate Formal concentration of [A-]: A- is no longer 0.02000 M, diluted by the addition of NaOH Initial volume of HA 50.00 F' 0.02000 M 0.0167 M 50 . 00 10 . 00 Initial Total volume concentration Dilution factor of HA Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Region 3: At the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Calculate [OH-]: Kw 1 10 14 x2 8 Kb 1 . 86 10 Fx Ka 10 6.27 x2 1.86 10 8 x [OH - ] 1.76 10 -5 M 0.0167 x Calculate pH: 1 10 14 log 0.0167 9.25 pH at equivalence point is not 7.00 pH will always be above 7.00 for titration of a weak acid because acid is converted into conjugate base at the equivalence point K pH - log[H ] log w x Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Region 4: After the Equivalence Point Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Adding NaOH to a solution of A- NaOH is a much stronger base than ApH determined by excess of OH- Calculate volume of excess OH-: Amount of added NaOH is 10.10 mL with equivalence point is 10 mL Vadded Vequivalence 10.10 10.00 0.10 mL Calculate excess [OH-]: Volume of excess OH- 0.10 [OH- ] 0.1000 M 1.66 10 4 M 50.00 10.10 Initial Total volume Dilution factor concentration of OHCalculate pH: 1.00 10 14 Kw 10.22 pH - log[H ] log log 1.66 10 4 [OH ] Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Titration Curve Titration of 50.00 mL of 0.02000 M MES with 0.1000 M NaOH Two Important Features of the Titration Curve Equivalence point: [OH-] = [HA] Steepest part of curve Maximum slope Maximum Buffer Capacity pH=pKa Vb = ½Ve Minimum slope Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Titration Curve Depends on pKa or acid strength Inflection point or maximum slope decreases with weaker acid - Equivalence point becomes more difficult to identify weak acid small slope change in titration curve Difficult to detect equivalence point Strong acid large slope change in titration curve Easy to detect equivalence point Acid-Base Titrations Titration of Weak Acid with Strong Base 5.) Titration Curve Depends on acid concentration Inflection point or maximum slope decreases with lower acid concentration - Equivalence point becomes more difficult to identify Eventually can not titrate acid at very low concentrations High Lowconcentration concentrationlarge smallslope slopechange changeinin titration titrationcurve curve Easy to detect equivalence point Difficult to detect equivalence point At low enough concentration, can not detect change Acid-Base Titrations Titration of Weak Base with Strong Acid 1.) Simply the Reverse of the Titration of a Weak Acid with a Strong Acid Again, Titration Reaction Goes to Completion: Again, Four Distinct Regions to Titration Curve Before acid is added just weak base reaction - pH determined from Kb Kb F-x x x Before equivalence point, buffer - pH determined from Henderson Hasselbach equation - Va=½Ve then pH = pKa (for BH+) pKa and pKb can be determined from titration curve [ A ] pH pK a log [HA] Acid-Base Titrations Titration in Diprotic Systems 1.) Principals for Monoprotic Systems Apply to Diprotic Systems Multiple equivalence points and buffer regions Multiple Inflection Points in Titration Curve Two equivalence points Kb1 Kb2 Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Titration of 10.0 mL of 0.100 M base (B) with 0.100 M HCl - pKb1 = 4.00 and pKb2 = 9.00 Volume at First Equivalence Point (Ve) Ve ( mL )0.100 M 10.00 mL ( 0.1000 M ) Ve 10.00 mL mmol of HCl mmol of B Volume at Second Equivalence Point Must Be 2Ve - Second reaction requires the same number of moles of HCl Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Point A - Before Acid Added Weak base problem Kb1 0.100 - x x x x2 1.00 10 4 x 3.11 10 3 0.100 x [H ] Kw pH 11.49 x Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Point between A &B - Before First Equivalence Point Buffer problem Kw 1 10 14 10 K a2 1 10 K b1 1 10 4 Point (1.5 mL) is before first equivalence point (10 mL) [ B] [ BH ] 10.00 ml 1.5 ml 8.5 5.67 1.5 ml 1.5 [ B] 10.00 log 5.67 10.75 pH pKa 2 log [ BH ] Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Point B - Before First Equivalence Point Buffer problem Kw 1 10 14 10 K a2 1 10 K b1 1 10 4 Point B (5 mL) is halfway to first equivalence point (10 mL) [B] [BH ] 10.00 ml 5 ml 5 1 5 ml 5 [B] 10.00 log 1 10.00 pH pK a2 log [BH ] pH = pKa2=10.00 Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Point C - [H ] First Equivalence Point Intermediate form of the Diprotic acid K 2 K1F K1Kw K1 F Account for dilution for formal Solve for pH using concentration (F) of BH+ intermediate form equation Initial volume of B 10.00 5 10 5 F' 0(.10 1000 M 0 )( 10 )( 0.0500 ) ( 10 )( 1014 ) .0500 M [H ] 3.16 x10 8 1010 5 .00 10.00 0.0500 8 Initial pH log( 3.16 x10 ) 7.50 Total volume concentration Dilution factor of B Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Point D - Before Second Equivalence Point Buffer Problem Kw 1 10 14 5 K a2 1 10 K b1 1 10 9 Point D (15 mL) is halfway to second equivalence point (2x10 mL). First, subtract Ve (10 mL) [BH ] 2 [BH2 ] 10.00 ml 5.00 ml 5 1 5 ml 5 [BH ] 5.00 log 1 5.00 pH pK a2 log [BH 2 ] 2 pH = pKa1=5.00 Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Point E - Second Equivalence Point Weak acid problem Account for dilution for formal Initial volume of B concentration (F) of BH2+2 10.00 F' 0.1000 M 0.0333 M 20 . 00 10 . 00 Initial Total volume concentration Dilution factor of B pH determined by acid dissociation of BH2+2 Kb2 Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case 0.0333 - x Point E - Second Equivalence Point Weak acid problem Ka1 K a1 x x x2 x2 1.0 10 5 F x 0.0333 x x 5.72 10 4 pH 3.24 Kw K b2 Acid-Base Titrations Titration in Diprotic Systems 2.) A Typical Case Beyond Point E - Past Second Equivalence Point Strong acid problem pH from volume of strong acid added. Addition of 25.00 mL: Excess acid: Vadded Vequivalence 25.00 20.00 5.00 mL Concentration of H+: 5.00 2 [H ] 0.1000 M 1.43 10 M 25.00 10.00 pH: pH - log [H ] log( 1.43 10 2 ) 1.85 Acid-Base Titrations Titration in Diprotic Systems 3.) Blurred End Points Two or More Distinct Equivalence Points May Not be Observed in Practice - Depends on relative difference in Kas or Kbs - Depends on Relative strength of Kas or Kbs Only one Equivalence point is clearly evident Second Ka is too strong and is not a weak acid relative to titrant Acid-Base Titrations Titration in Diprotic Systems nd derivative is zero End point: End point: 1st2derivative is maximum 4.) Using Derivatives to Find End Point Useful when End points overlap End Point of titration curve is where slope is greatest - Dph = 4.400-4.245=0.155 dpH/dV is large DpH change in pH between consecutive points DV average of pair of volumes Second derivative is similar difference using first derivative values Acid-Base Titrations Titration in Diprotic Systems 5.) Using Gran Plot to Find End Point Method of Plotting Titration Data to Give a Linear Relationship A graph of Vb10-pH versus Vb is called a Gran plot Vb 10 pH HA K a Ve Vb A where: Vb = volume of strong base added Ve = volume of base needed to reach equivalence point A-, HA = activity coefficients ≈ 1 Acid-Base Titrations Titration in Diprotic Systems 5.) Using Gran Plot to Find End Point Plot is a straight line - If ratio of activity coefficients is constant Slope = -KaHA/aX-intercept = Ve (must be extrapolated) Measure End Point with data Before Reach End Point Only use linear region of Gran Plot - Changing ionic strength changes activity coefficients added salt to maintain constant ionic strength Slope Gives Ka x-intercept gives Ve Never Goes to Zero, approximation that every mole of OH- generates one mole of A- is not true as Vb approaches Ve Acid-Base Titrations End Point Determination 1.) Indicators: compound added in an acid-base titration to allow end point detection Common indicators are weak acids or bases Different protonated species have different colors Acid-Base Titrations End Point Determination 1.) Indicators: compound added in an acid-base titration to allow end point detection Color Change of Thymol Blue between pH 1 and 11 pK = 1.7 pK = 8.9 Acid-Base Titrations End Point Determination 2.) Choosing an Indicator Want Indicator that changes color in the vicinity of the equivalence point and corresponding pH The closer the two match, the more accurate determining the end point will be Bromocresol purple color change brackets the equivalence point and is a good indicator choice Bromocresol green will change color Significantly past the equivalence point resulting in an error. Acid-Base Titrations End Point Determination 2.) Choosing an Indicator The difference between the end point (point of detected color change) and the true equivalence point is the indicator error Amount of indicator added should be negligible Indicators cover a range of pHs Acid-Base Titrations End Point Determination 3.) Example: a) What is the pH at the equivalence point when 0.100 M hydroxyacetic acid is titrated with 0.0500 M KOH? b) What indicator would be a good choice to monitor the endpoint?






