HEMOSTASIS
advertisement

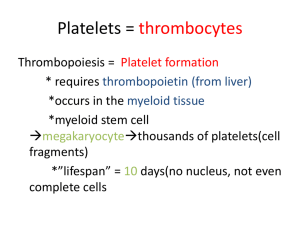
PLATELETS, HEMOSTASIS & BLOOD COAGULATION Learning objectives • To know the role of platelets in hemostasis • To understand the steps of hemostasis • To know the pathways of coagulation or clotting • To be familiar with some abnormal clotting conditions PLATELETS Hemopoiesis Platelets (Thrombocytes) • Thrombocytes: • Cell fragments shed from megakaryocytes – Lack nuclei – Have organelles and cytosolic enzymes for generating energy and synthesizing secretory products – High concentrations of actin and myosin • Remain functional for an average of 10 days • Removed from circulation by tissue macrophages • Do not leave blood as WBCs do – About ⅓ are stored in blood-filled spaces in spleen – Released when needed by sympathetically induced splenic contraction • Thrombopoietin: – Hormone produced by liver, -- increases number of megakaryocytes and therefore increases platelet production Hemostasis • Prevents blood loss from a broken blood vessel • Involves 3 major steps 1) Vascular spasm Reduces blood flow through a damaged vessel 2) Formation of a platelet plug Platelets aggregate on contact with exposed collagen in damaged wall of the vessel Platelets release ADP which causes surface of nearby circulating platelets to become sticky in order to adhere to first layer of aggregated platelets 3) Blood coagulation (clotting) Transformation of blood from liquid into a solid gel Much rarer occurrence of bleeding from mediumto large-size vessels usually cannot be stopped by the body’s hemostatic mechanisms alone. Bleeding from a severed artery is more profuse and therefore more dangerous than venous bleeding. First-aid measures for a severed artery include applying external pressure to the wound. Hemorrhage from a traumatized vein can often be stopped simply by elevating the bleeding body part or applying mild external compression is usually adequate. VASCULAR SPASM (Vasoconstriction): A cut or torn blood vessel immediately constricts. Vascular spasm reduces blood flow through an injured vessel by a paracrine released locally from (endothelium) of the injured vessel. This constriction, or vascular spasm, slows blood flow through the defect and thus minimizes blood loss. Also the opposing endothelial surfaces of the vessel are pressed together and adhere to each other, further sealing off the damaged vessel. These physical measures alone cannot completely prevent further blood loss, but they minimize blood flow through the break in the vessel until the other hemostatic measures can actually plug up the hole. HEMOSTSTIC PLATELET PLUG Platelets aggregate to form a plug at a vessel tear or cut. Platelets normally do not stick to the smooth endothelial surface of blood vessels, but when this lining is disrupted because of vessel injury, platelets adhere to and are activated by the exposed collagen. When platelets passing by in the blood are exposed to collagen, they become adhered to the collagen by means of integrin, This adhesion prevents these platelets from being swept forward in the circulation. This layer of stuck platelets forms the foundation of a hemostatic platelet plug at the site of the defect. Platelet plug • Thus formation of a platelet plug involves the three successive, closely integrated events of; • adhesion , • activation and • aggregation Functions of aggregated platelets : 1) Actin-myosin complex contract to strengthen the loose plug 2) Release several powerful vasoconstrictors eg. Serotonin, epinephrine, thromboxane A2 3) Platelet plug release chemicals like platelet factor3 (PF3) enhancing blood clotting CLOT FORMATION CLOTTING FACTORS • • • • • • • • • • • • • • Factor I = Fibrinogen Factor II = Prothrombin Factor III = Tissue factor Factor IV = Calcium Factor V = Labile factor Factor VI - Does not exist Factor VII = Stable factor Factor VIII = Antihemophilic factor A Factor IX = Antihemophilic factor B or Christmas factor Factor X = Stuart Prower factor Factor XI = Antihemophilic factor C Factor XII = Hageman factor Factor XIII = Fibrin stabilising factor Clot Formation • Reinforces platelet plug and converts blood in the vicinity of vessel injury into a non flowing gel • Clotting factors are always present in blood plasma in inactive precursor form – Vessel damage that exposes collagen initiates cascade of reactions that involve successive activation of clotting factors , finally • Convert fibrinogen fibrin by means of the intrinsic clotting pathway The original fibrin web is rather weak, because the fibrin strands are only loosely interlaced. However, chemical linkages rapidly form between adjacent strands to strengthen and stabilize the clot meshwork. This cross-linkage process is catalyzed by a clotting factor known as factor XIII (fibrin-stabilizing factor), which normally is present in the plasma in inactive form. Fibrin is the stretchiest natural protein. This highly elastic property accounts for the extraordinary stretchiness of blood clots. On average fibrin fibers can be passively stretched to 2.8 times their original length and still snap back to their starting length and can be stretched to 4.3 times their original length before they break. Clotting Cascade • Series of steps involving 12 plasma clotting factors that lead to final conversion of fibrinogen into a stabilized fibrin mesh • May be triggered by – Intrinsic pathway • Involves seven separate steps • Set off when factor XII (Hageman factor) is activated by coming into contact with exposed collagen in injured vessel or foreign surface such as glass test tube. – Extrinsic pathway • Requires only 4 steps • Requires contact with tissue factors external to the blood • Tissue thromboplastin (factor III) released from traumatized tissue activate factor VII. Tissue thromboplastin and activated factor VII activates factor X. Clot Pathways CLOT RETRACTION • Once a clot is formed, contraction of platelets within the clot shrinks the fibrin mesh, bringing the edges of damage vessel close to each other. • During clot retraction, fluid is squeezed from the clot, thus making it semisolid & more strong. • This fluid is SERUM, which is plasma minus fibrinogen and other clotting factors that have been removed during clotting. Role of thrombin FIBRINOLYSIS • Fibrinolysis: • means dissolution of excess of clot • to prevent blockage of small blood vessels Abnormal Blood Clotting • • • • Thrombus – Abnormal intravasculaar clot attached to a vessel wall Emboli – Freely floating clots Factors that can cause thromboembolism – Roughened vessel surfaces associated with atherosclerosis – Imbalances in the clotting-anticlotting systems – Slow-moving blood – Occasionally triggered by release of tissue thromboplastin into blood from large amounts of traumatized tissue EXCESSIV BLEEDING: Hemophilia – Excessive bleeding caused by deficiency of one of the factors in the clotting cascade (factor VIII or IX) – Hemophilia A - Def. of factor VIII – Hemophilia B - Def. of factor IX PURPURA • occurs due to decrease in no. of platelets(thrombocytopenia) • Small pin point or dot capillary hemorrhages are visible in the skin • Vitamin K deficiency can cause bleeding tendency, due to incomplete activation of vit. K dependent factors (factors II, VII, IX and X) HEMOPHILIA Hemarthrosis THANKS WITH BEST WISHES