File
advertisement

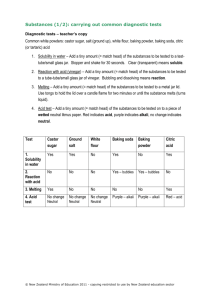
Done By: Tanya R. 8E Submitted To: Ms. Jocelyn K. Experiment #1: Red Cabbage Indicator Introduction: In this experiment, we are going to make an indicator and observe how its color changes when it is mixed with different substances from around the house. The color change of the indicator tells whether the substance is an acid, a base, or neutral. If a substance is an acid, it will turn pink and then red when it is mixed with the indicator. If it is a base, it will turn blue, then green, then yellow. If it is neutral, it will not change its color. The pH scale is a scale that usually runs from 1 to 14. A strong acid may have a pH of 1-2, while a strong base may have a pH of 13-14. A pH near 7 is neutral. When an acid is mixed with a base in equal amounts, salt and water is produced, making the solution neutral. Materials: 1 Red cabbage Plastic cups Cooking pot Ladle / large spoon Baking soda Salt Hot sauce Vinegar Sprite (or other soft drinks) Rubbing alcohol Window cleaner Liquid soap Optional: other powder or liquid items around the house Procedure: 1. Fill the cooking pot about halfway with water. 2. Take the red cabbage, and start pulling off parts of its leaves. 3. Put the leaves inside the pot. Fill up the pot until all of the leaves of the cabbage are covered with water. 4. Put the pot on the stove, and turn up the heat. Let the water boil. 5. After about 10 minutes, turn the heat off. Let the water cool down for 15-20 minutes. 6. Observe the cabbage. The cabbage loses a lot of its color, which is now in the water. 7. Using a ladle or a large spoon, take out the leaves of the cabbage and leave only the purple-dyed water behind. Now you have your indicator. 8. It’s time to test your materials to see if they are acids, bases, or neutral. Let’s start with baking soda. Put some in a cup. 9. Pour some of your indicator in the cup. The liquid turns green. What can you say about the substance? 10. Now let’s try repeating the same procedure and using salt instead of baking soda this time. The liquid stays purple. 11. Next, let’s use hot sauce. The indicator turns red. 12. Now let’s use vinegar and see the result. Once again, the solution turns bright red. 13. Let’s use Sprite next. A third time, the indicator turns red. 14. Next, let’s use rubbing alcohol. The indicator does not change its color. 15. Now let’s try window cleaner. The solution becomes green. 16. Lastly, we’ll use liquid soap. The indicator turns a bright pinkish red color. 17. Now we have many different liquids that resulted when household items were mixed with red cabbage juice. 18. Now, let’s try to neutralize a solution by mixing an acid and a base together. Recall from earlier that mixing an acid and a base in equal amounts creates a neutral. Let’s mix baking soda and vinegar together. The solution bubbles up. 19. Pour the indicator into the solution. 20. Observe. The solution turns a bluish color. Is it neutral? Result and Observation: Here I recorded my results and what I can infer about the items that I used. Substance Color when mixed with indicator Acid, Base, or Neutral? Baking soda Green Base Salt Purple Neutral Hot sauce Red Acid Vinegar Bright red Acid Sprite Red Acid Rubbing alcohol Purple Neutral Window cleaner Green Base Liquid soap Bright pinkish red Acid Baking Soda + Vinegar (unequal amounts) Dark blue Base The solution of baking soda and vinegar didn’t neutralize. My inference can be that I didn’t put the two substances in equal amounts, making the solution basic. Conclusion: In this experiment, I learned about acids and bases. I made an indicator to see what substances are acids, which substances are bases, and which are neutral. I got a variety of different colors. Now I know the properties of the substances around the house. I can predict how they can be helpful and how they can be harmful now that I know whether they are acids, bases or neutral. Also, I tried to neutralize a solution by mixing an acid and a base. I got a basic solution as a result of mixing too much of a basic substance than an acidic one.