Click on image to content
advertisement

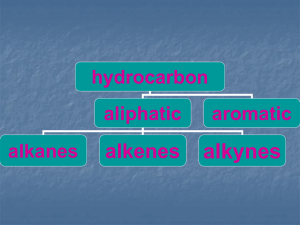
CHEMISTRY OF METHANE INTRODUCTION Molecular formula = CH4 Molecular mass = 16 Empirical formula = CH4 Empirical formula mass = 16 State: Gas at room temperature. Occurance: marsh, stagnant ponds. It is the major constituent of natural gas. Natural gas contains 94.6% methane. STRUCTURE OF METHANE Composition of methane molecule: Methane molecule consists of one carbon and four hydrogen atoms (CH4). Geometry of methane: Methane molecule is tetrahedral in structure in which carbon is central atom and four H-atoms are surrounding it in three-dimensions. Bond Angles: HCH-bond angles are 109.5o. Bond Length: All C-H bonds are 1.09Ao. METHOD OF PREPARATION OF METHANE BY THE CATALYTIC REDUCTION OF METHYL IODIDE: CH3-I + H2 CH4 +HI BY THE HYDROLYSIS OF ALUMINIUM CARBIDE: In laboratory methane can be prepared by boiling aliminium carbide with water. Al4C3 + 12H2O 3CH4 +Al (OH)3 CHEMICAL PROPERTIES OF METHANE COMBUSTION REACTION: Combustion of methane is an exothermic reaction in which a large amount of energy is liberated. Due to this property, methane is used as a domestic and industrial fuel. CH4 + 2O2 CO2 + 2H2O HALOGENATION: Replacement of halogen atom with H-atom of an organic compound is called Halogenation. It is a substitution reaction.This reaction occurs in the presence of sun light.It is a free radical mechanism reaction.The reaction will continue till the replacement of all four hydrogen atoms of methane with chlorine. CHLORINATION: CH4 + Cl2 CH3Cl + Cl2 CH2Cl2 + Cl2 CHCl3 + Cl2 CH3Cl + HCl (chloro methane) CH2Cl2 +HCl (dichloro methane) CHCl3 + HCl (chloroform) CCl4 +HCl (carbon tetra chloride) OUTPUT: Since it is a chain reaction, therefore, it gives a mixture of different compounds. PHYSICAL PROPERTIES OF METHANE Methane is a colorless, odourless and nonpoisonous gas. Melting point = -182.5oC. Boling point = -169.5oC. Its molecule is symmetrical. It is lighter than air. It is slightly soluble in water but fairly soluble in ether and alcohol. USES OF METHANE Domestic and industrial fuel. Shoe polish. Printing ink. Tyre manufacturing. Manufacture of methyl alcohol.