File
advertisement

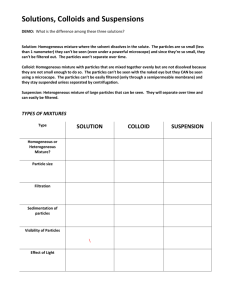
Mid-semester Examination Semester 1 Academic Year 2014 Course Code: SC21101 Subject: Science 1 Matthayomsuksa 1 Scores: 20 Marks Time: 60 minutes DIRECTIONS: 1. This examination paper consists of 50 items. 2. Mark the correct answer on the answer sheet. 3. Hand in the examination paper and the answer sheet to the proctors. MULTIPLE-CHOICE TEST 1. How does the knowledge of science be applied in terms of technology? 1. When a student wakes up early in the morning and arrives at school early. 2. When a glass of cold water undergo change of state like condensation. 3. When a home appliance is created to make life easier and comfortable. 4. All of the above. 2. In a series of steps done in scientific investigation, after we arrive in making conclusion what will be the next thing to do? 1. We should start formulating a hypothesis. 2. We should start analyzing results. 3. We should be able to identify the problem. 4. We should start writing a report. 3. In the field of specialization, what does meteorologist do? 1. Meteorologist studies rocks and minerals. 2. Meteorologist studies planets. 3. Meteorologist studies the interactions between living things and the environment. 4. Meteorologist studies weather. Convert numbers 4 to 7 to the given units and choose the best answer. 4. What is 28.4 centimeter to millimeter? 1. 284 mm 2. 2.84 mm 3. 2840 mm 4. 0.284 mm 5. What is 0.79 meter to centimeter? 1. 0.079 cm 2. 79 cm 3. 790 cm 4. 7900 cm 6. What is 7.32 kilogram to gram? 1. 0.732 g 2. 73.2 g 3. 732 g 4. 7320 g 7. What is 3.4 gram to milligram? 1. 0.34 mg 2. 34 mg 3. 340 mg 4. 3400 mg 8. Which is NOT a scientific phenomenon? 1. Siwat’s ice-cream is melting because it’s sunny. 2. Many of the students in EP got sweat when the air-conditioner was not working. 3. Mr. David was glad because the EP students got higher score in their Math test. 4. The bees went inside the classroom because some students were drinking fruit-juice in their classroom. Continue Page 2 Mid-semester Examination Science 1 (SC21101) Matthayomsuksa 1 2014 9. The amount of matter in an object is called what? 1. Weight 2. Mass 3. Kilograms 4. Newton 10. The gravitational force acting on the object is called what? 1. Weight 2. Mass 3. Kilograms 4. Newton 11. Which of the following laboratory apparatus is use for transferring small amounts of solids? 1. Test tube 2. Measuring Cylinder 3. Spatula 4. Crucible 12. Which of the following laboratory apparatus is use for measuring volumes of liquids? 1. Test tube 2. Measuring Cylinder 3. Spatula 4. Crucible 13. If you are ask to make something that would help to increase the human life span, what appropriate invention you are going to make? 1. Create something about laser surgery. 2. Develop new building techniques. 3. Invent new medicines and treatments for diseases 4. Invent better strains of rice plants 14. Radium, Uranium, and Plutonium are what? 1. Flammable 2. Radioactive 3. Corrosive 4. Explosive 15. In Scientific Investigation, how would we be able to determine what we want to find out? 1. We should make a conclusion right away. 2. Analyzing and interpreting the data of our experiment would help us. 3. We should start first with identifying the problem. 4. Forming a hypothesis is the key to determine what we want to find out. 16. Why do you think the amount of mass is constant in different places and weight is not? 1. It is because mass and weight measuring instruments are not the same. 2. It is because our moon is smaller compare to our planet earth. 3. It is because weight is measured by gravitational force acting on the object. 4. It is because mass is measured by gravitational force acting on the object. 17. The SI Unit value of Micro is what? 1. 0.1 2. 0.01 3. 0.001 4. 0.000 001 18. The SI Unit value of Giga is what? 1. 1000 2. 1 000 000 3. 1 000 000 000 4. 1 000 000 000 000 19. Area is equals to what? 1. length x height 2. length x width 3. height x width 4. length x height x width 20. Calculate the volume of the object below and choose the correct answer: 1. 26 cm 2. 52 cm 3. 576 cm 4. None of these is correct 21. What instrument do we have to use when we measure the internal and external diameters of a test tube? 1. Meter Ruler 2. Vernier Caliper 3. Graduated Cylinder 4. Beam Balance 22. In measuring the level of liquid, why it is so important to check the meniscus? 1. It is important because some measuring apparatus have concave or convex based. 2. It is important because gravitational force is not constant in all places. 3. It is important because beaker and graduated cylinder are different apparatus for measuring liquid 4. None of them is correct. 23. The SI Unit for Temperature is what? 1. Celsius 2. Fahrenheit 3. Romer 4. Kelvin 2 Continue Page 3 Mid-semester Examination Science 1 (SC21101) Matthayomsuksa 1 2014 Figure 1 shows an outline of a leaf which has been traced on a graph paper. Answer numbers 24-26. 24. What is total number of completed squares, half-completed squares, and more-than-halfcompleted squares? 1. 11 2. 12 3. 13 4. 14 25. What is the area of the leaf? 1. 11 cm² 2. 12 cm² 3. 13 cm² 4. 14 cm² 26. What is the area of each square? 1. 0.5 cm² 2. 1 cm² 3. 2 cm² 4. 4 cm² 27. Which of the following science process skills is used in making conclusions? 1. Predicting 2. Classifying 3. Analyzing Data 4. Controlling Variables 28. What does a scientist do while making a hypothesis? 1. State an outcome of a future event 2. Make a smart guess to explain a problem 3. Find a meaning to the observations 4. Make general statement about a group of observations 29. If the volume of the water in a graduated cylinder is 25 cm³ before the stone was added, then what is the volume of the stone if the volume goes up to 38 cm³ after the stone was added into the water? 1. 13 cm³ 2. 14 cm³ 3. 15 cm³ 4. 16 cm³ 30. 30cmᵌ of water is poured into a measuring cylinder. One screw can displace 2.5 cmᵌ of water. How many more screws should be put into the measuring cylinder so that the level of water reaches 50 cmᵌ? 1. 7 2. 8 3. 9 4. 10 31. It is almost everything around us that has mass and occupies space. 1. Element 2. Matter 3. Metal 4. Solution 32. What substance that consists of only one type of particle? 1. Solution 2. Mixture 3. Element 4. Compound 33. A matter that is formed when two or more substances combine chemically in a chemical reaction 1. Solution 2. Mixture 3. Element 4. Compound 34. Red blood cells, white blood cells, and plasma in the blood are examples of what? 1. Mixture and Compound 2. Element only 3. Mixture only 4. Compound only 35. Which of the following is NOT a characteristic of a solid? 1. Particles are very close together 2. Spaces between particles are very big 3. Particles are fixed, and have regular pattern 4. Particles vibrate about their positions 36. What is matter made up of? 1. Atoms 2. Solid 3. Compound 4. Elements 37. If the energy is sufficient, what will change in the matter? 1. Composition 2. State 3. Classification 4. Chemical Content 38. When ice is heated it will change into what physical state? 1. Solid 2. Plasma 3. Liquid 4. Gas 3 Continue Page 4 Mid-semester Examination Science 1 (SC21101) Matthayomsuksa 1 2014 39. When drinking water is added with heat energy it will change into what physical state? 1. Solid 2. Plasma 3. Liquid 4. Gas 40. How do we call the process where liquid becomes gas? 1. Condensation 2. Sublimation 3. Evaporation 4. Deposition 41. How do we call the process where ice directly becomes gas? 1. Condensation 2. Sublimation 3. Evaporation 4. Deposition 42. Which of the following is matter? 1. Ink 2. Heat 3. Light 4. Sound 43. What group of elements that have some properties of both at different conditions? 1. Metalloids 2. Metals 3. Non-metals 4. Mixtures 44. What group of elements that are good conductors? 1. Metalloids 2. Metals 3. Non-metals 4. Mixtures 45. Which of the following is NOT a characteristic of a liquid? 1. Particles are close together 2. Spaces between particles are small 3. Particles move freely and collide into each other at high speeds 4. Particles are not arranged in a regular pattern 46. Which of the following is NOT a characteristic of a metal? 1. Surface Appearance is Shiny 2. Ductile and Malleable 3. Good Conductors of Heat and Electric 4. Low Melting and Boiling Point 47. Which of the following is Mercury can be used? 1. Making lead of pencil 2. Packaging beverage cans 3. Making thermometer to measure temperature 4. Making pipe for water supply 48. What method is used to separate liquids of different boiling temperature? 1. Distillation 2. Evaporation 3. Sieving 4. Filtration 49. What is the difference between a solution and a suspension? 1. A solution is clear and a suspension will settle down after a while. 2. A solution is a mixture and a suspension is an element. 3. A solution has larger particles than a suspension. 4. A solution needs heat and a suspension removes heat. 50. In your experiment, what happened to the temperature of boiling water when you continue to add energy through heating? 1. The temperature remains constant. 2. The temperature continues to go up more than 100°C. 3. The temperature will go down once it reaches 95°C. 4. None of these is correct. -End- 4 Mid-semester Examination Science 1 (SC21101) ANSWER KEY 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 3 4 4 1 2 4 4 3 2 1 3 2 3 2 3 3 4 3 2 3 2 1 4 3 3 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 5 2 3 2 1 2 2 3 4 3 2 1 2 3 4 3 2 1 1 2 3 4 3 1 1 1 Matthayomsuksa 1 2014 Mid-semester Examination Science 1 (SC21101) 6 Matthayomsuksa 1 2014