Carbohydrates are - Issaquah Connect
advertisement

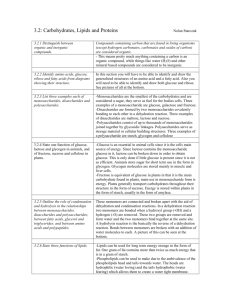
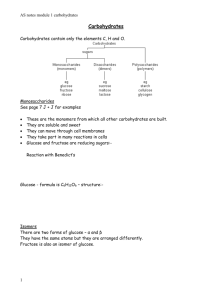
Introduction to Organic Chemistry & Carbohydrates Note Templates pgs. 41-43 Before we start… 1. 2. 3. To be considered a living organism, you must be made up of _____. What is inside of cells? What are those things made up of? Meet the Elements ► Watch for what elephants are made of… What do I want you to know from Study Guide 2.1 and 2.2? ► 2.1 Atoms, Ions, Molecules ► Atom structure (basic) ► Compound ► Ion ► Bonds: Ionic (transfer) Covalent (share) ►Molecule What do I want you to know from Study Guide 2.1 and 2.2? ► 2.2 Properties of Water ► Polar Molecule “Like dissolves like” ► Hydrogen Bond Organic Chemistry ►The study of carbon-containing compounds and their properties. ►Biochemistry: Made by living things All contain the elements carbon and hydrogen Essential Elements ►30 elements essential to human life. Element O C H N Ca P K, Cl, S % by mass in body 65 18 10 3 1.5 1.2 0.2 each Essential Elements ►30 elements essential to human life. Some will make a slightly different list: CHOPKINS CaFe Mg This sounds like someone describing an excellent small restaurant. Say it out loud This list is similar to the first list with some things added and some things missing. Inorganic compounds: ► Inorganic: All other compounds Usually do not contain carbon H2O Ca3(PO4)2 NaCl Carbon containing molecules not considered organic: ►CO2 ►Bicarbonate ions HCO3- Main branches of Organic Chemistry: ►Biochemistry, which includes: Carbohydrates ►Simple and complex sugars Proteins Lipids ►Fats and oils Nucleic acids ►DNA & RNA Organic Chemistry Carbon ► Always forms 4 bonds (to fill the outer shell to 8 electrons to become stable) These carbons, in nature, will link with each other to form chains or rings ► Make large complex molecules called MACROMOLECULES ► ► Hydrocarbon examples: C-C-C-C-C C=C Can you fill in these molecules with the necessary hydrogen atoms? Remember Carbon needs 4 bonds but hydrogen only needs one. Hint: The first compound needs 12 H atoms and the second needs 4 H atoms . 22_502 H H H H C H C H Breaking Down and Combining Molecules A. Dehydration Synthesis (Condensation) Forming bigger molecules B. Hydrolysis Breaking bigger molecules into smaller molecules Dehydration Synthesis (Condensation): ► Dehydration- To take out water (H2O) ► Synthesis To create (To put together, to make bigger) H2O Condensation + H2O Example: Monosaccharide + Monosaccharide Disaccharide + H2O ► Glucose + Glucose C6H12O6 Maltose + H2O C6H12O6 C12H22O11 Hydrolysis: ► Hydro- water (H2O) ► Lysis- to split Example: Disaccharide + H2O Monosaccharide + Monosaccharide ► Sucrose + Water Glucose + Fructose C24H22O11 + H2O C6H12O6 + C6H12O6 Welcome to: Mmmmm -- so sweet and yet so starchy… Carbohydrates are: Sugars Sugars end with suffix “-ose” Produced by photosynthesis Contain the elements: C, H, O or in the ratio of C(H2O) Monosaccharides: Simple sugars Ex. Glucose C6H12O6 * very, very important! • It is from this molecule that animals get their energy Ex. Fructose C6H12O6 “1” All C6H12O6 Notice that glucose and fructose have identical formulas but their structures are different. Disaccharides “2” Two monosaccharides joined together through dehydration synthesis glucose – glucose = maltose glucose – fructose = sucrose glucose – galactose = lactose Polysaccharides “many sugars” Complex carbohydrates Long chains of monosaccharides Also joined through dehydration synthesis Two basic types and purposes of Polysaccharides: • Storage, used to store energy • Structural, for support and composition Storage Carbohydrates Starch: complex, stores energy in plants “pearl necklace, each pearl = glucose” broken down into monosaccharides glu-glu-glu-glu-glu-glu-glu-glu Storage Carbohydrates Glycogen – a branched chain of as many as 2000 glucose units. Storage form of glucose in animals. Structural Carbohydrates Cellulose: • • • • Structural compound in plant cell walls • found in wood and paper uses a modified form of glucose still a “string of pearls” cannot be broken down by the body Cellulose Structural Carbohydrates Chitin: • • • Structural carbohydrate in insect exoskeletons and fungal cell walls uses a highly modified form of glucose still a “string of pearls” Comparison: Starch vs. Cellulose Identical in composition but not quite in structure. • • The bond joining the glucose units together are different….our bodies can break the bond in starch/amylose to get at the individual glucose units but not in cellulose. That’s why we can eat potatoes and pasta but not woody material. Source:http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb1/part2/sugar.htm We tend to use the words “sugar” and “starch” and “carbohydrate” loosely. When we say a food is “high in carbohydrates” do we mean the food is high in “sugar” or “starch” or both? Potatoes and bread “starchy” foods and are “high in carbohydrates” due to the starch they contain. Fruits are “high in carbohydrates” but we would never say they were “starchy” - they contain almost all simple carbohydrates such as sucrose, fructose. We tend to say “high in carbohydrates” for foods with a high level of the complex carbohydrates known as starch. And for foods that have high levels of the simplex carbohydrates, we tend to say they are “high in sugar”. But sugar and starch are really the same thing! They’re both made from glucose. The end