COX-2 - PCMA

NSAIDs: Treatment and Pitt falls
Jacques R Snyman (MD)
NSAIDs
• Classified as non-selective nsNSAIDS and
Selective NSAIDs
– nsNSAIDs are the typical “old fashioned” ibuprofen, diclofenac, naproxen etc which blocks the cyclo-oxygenase enzymes 1 and 2
– Selective NSAIDs include (higher affinity for COX 2)
• Coxibs such as celecoxib, etoricoxib, etc
• Meloxicam, nimesolide etc
Major Pathways
NSAID Physiology
• The physiologic action of COX-1 includes the normal, physiological production of prostanoids, which are involved in the maintenance of the gastrointestinal (GI) mucosa and platelet aggregation:
• Inhibition of this constitutively expressed enzyme results in e.g.:
• decreased mucus and bicarbonate production from the GI mucosa.
• reduced mucosal blood flow and inhibition of epithelial proliferation.
• inhibition of coagulation
• inhibition of inflammation
• shifts renal medullary perfusion
NSAID Physiology
• COX-2 is generally an inducible enzyme released by cytokines, mitogens and endotoxins in inflammatory cells and is also responsible for prostaglandin production in inflamed tissues:
– selective inhibition of this enzyme results in
• relief of pain and inflammation with a mitigated risk of gastrointestinal complications, (this led to the development of the coxibs)
• COX-2 inhibition results in reduced tumor genesis, especially development of colorectal adenomas
• shifts renal blood flow (influences auto-regulation in the stressed kidney)
Prostaglandin inhibitory activity correlates to anti-inflammatory effect
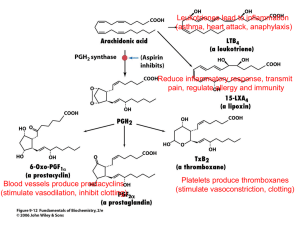
NSAID: MECHANISM OF ACTION
Non-steroidal anti-inflammatory drugs (NSAIDs)
• All NSAIDs inhibit the cyclooxygenase required for conversion of arachidonic acid to endoperoxide intermediate (PGG2 and
PGH2).
• NSAIDs inhibit prostaglandin and thromboxane synthesis, they are potent inhibitors of cyclooxygenase and eliminate all prostaglandins and thromboxanes in every cell they reach
• Prostaglandins and thromboxanes play crucial roles in:
– Pain
– Inflammation
– Fever
– Excessive blood clotting
What are the risks?
NSAIDS AND THE HEART
Aspirin
• Aspirin is the prototype anticoagulant for cardiovascular protection (arterial side)
• Acetylates the COX 1 and 2 enzymes for the life span of the platelet (duration is dependant on new platelet production)
– Irreversible inhibition
– Endothelium escapes (COX produced by new coding from DNA)
– Platelet has no nucleus
– Vasodilatation and inhibition of coagulation is PGI
2 dependent from endothelium
Arachidonic acid metabolism via cyclooxygenase-1 and cyclooxygenase-2 in the cardiovascular system.
[Schrör et al. 2005].
Inflammation drives Plaque Rupture
Does it matter?
NSAIDS AND THE KIDNEY
NSAID Physiology (Kidney)
• COX-2 enzyme is expressed constitutively in the adult mammalian renal cortex, macula densa, thick ascending limb, interstitial cells in the inner medulla, and papilla and podocyte, likely responsible for the adverse cardio-renal effects seen with some COX-2 inhibitors [Nantel et al. 1999;
Guan et al. 1997; Komhoff et al. 1997; Harris and Breyer, 2001].
• Renal COX-2 expression has been found to be up-regulated in renal
ischemia and salt-depleted states, suggesting that COX-2 derived prostanoids may play an important role in maintaining medullary blood supply, salt excretion, and systemic blood pressure [Nantel et al. 1999;
Guan et al. 1997; Komhoff et al. 1997; Harris and Breyer 2001].
• Renin-release and renal prostacyclin synthesis have been demonstrated to be COX-2 dependent [Stichtenoth et al. 2005].
• Evidence suggests that specific COX-2 inhibition may induce renal ischemia, dyselectrolytemia, and an abnormal blood pressure response
[Harris et al. 1994], leading to sodium and fluid retention and a decreased GFR [Whelton et al. 2001; Catella- Lawson et al. 1999; Rossat
et al. 1999].
Who is at risk and why?
RISK
Adverse Cardiovascular Effects of NSAIDs: Driven by Blood
Pressure, or Edema?
Ashish Aneja MD; Michael E. Farkouh MD, MSc
Ther Adv Cardiovasc Dis. 2008;2(1):53-66.
• The harmful cardio-renal effects of some nsNSAIDs are well described and thought to be related to inhibition of prostanoid synthesis.
• COX-2 inhibitors promised and delivered a lower incidence of gastrointestinal side effects.
– However, the COX-2 inhibitors have not been found to be devoid of cardio-renal side effects. Indeed, some of these agents lead to increased blood pressure, an excessive risk of congestive heart failure and pro-thrombotic effects, especially in high risk populations.
– These deleterious effects, however, may not be class-specific and possibly related to pharmacokinetics, enzyme specificity and endothelium effects.
AEs: A class effect?
• However, these may not be class effects, at least in clinically used doses of all COX-2 inhibitors as shown in randomized trials and from epidemiological data.
– Some investigators have explained these differences by the degree and specificity of COX-2 inhibition and also by their metabolism and
pharmacokinetics [Warner et al.].
– Evidence suggests that rofecoxib has stronger COX-2 selectivity than others [Chang and Harris 2005] and this may account for the generally negative cardio-renal effects with this agent in multiple randomized and non-randomized studies.
– The COX-1 enzyme, present constitutively in the kidneys and appears
to play an important role in renal prostaglandin synthesis. The predominant renal prostaglandins are PGE2 and PGI2 of which PGE2
influences sodium resorption in the thick ascending loop of Henle and the collecting tubule. Prostaglandin E2 also antagonizes the
antidiuretic effect of vasopressin in the collecting tubule [Schlondorff
1993].
What is registered
PI
Common Warnings in Package Inserts
• patients with congestive heart failure (NYHA II – IV)
• established ischaemic heart disease, peripheral arterial disease and/or cerebrovascular disease
• perioperative analgesia in the setting of coronary artery bypass surgery (CABG)
COX and the Kidney
RENAL SIDE EFFECTS
Renal Side Effects of Etoricoxib
• Data from 4770 patients were included in this Meta-analysis.
– Most patients were women and most were white (68.0%, 83.3% respectively).
– The mean (SD) age at baseline ranged from 53.6 (12.1) to 62.2 (8.4) years.
– Overall, the incidence of renal AEs was low and generally similar between the active-treatment groups.
– In the placebo; etoricoxib 60-, 90-, and 120-mg; naproxen; and ibuprofen groups, the incidences of hypertension were 2.0%, 4.0%, 3.4%, 4.7%, 2.9%, and 6.6%, respectively
• The only significant difference found was the incidence of hypertension with etoricoxib 90 mg/d versus that with placebo (P = 0.001); however, the rates of hypertension observed with etoricoxib at any dosage were not clinically meaningfully different versus comparator NSAIDs.
• Based on this combined data review, the risks for renal AEs were generally similar to those found with the comparator NSAIDs : naproxen 1000 mg/d and ibuprofen
2400 mg/d.
Clin Ther. 2004;26:70-83.
Percent difference (95% Cl) versus placebo in incidence of investigator-reported hypertension adverse effects
Effects of Nonsteroidal Anti-Inflammatory Drugs on Urinary Sodium
Excretion, Blood Pressure, and Other Renal Function Indicators in Elderly
Subjects Consuming a Controlled Sodium Diet
Effects of Nonsteroidal Anti-Inflammatory Drugs on Urinary Sodium
Excretion, Blood Pressure, and Other Renal Function Indicators in Elderly
Subjects Consuming a Controlled Sodium Diet
• Multicenter, double-blind, randomized, placebo controlled, parallelgroup study assessed renal function during dosing with etoricoxib 90 mg daily, celecoxib 200 mg twice daily, and naproxen 500 mg twice daily:
– Male and female subjects 60 to 81 years old (n = 85), in sodium balance on a controlled, normal sodium diet, were treated for 15 days.
– There were no clinically meaningful between-treatment differences in urinary sodium excretion, creatinine clearance, body weight, or serum electrolytes during the 2 weeks of treatment.
– Etoricoxib and celecoxib had no effect on the urinary thromboxane metabolite, 11-dehydrothromboxane B2, while significantly decreasing the urinary prostacyclin metabolite, 2,3-dinor-6-keto
PGF1α.
• J. Clin. Pharmacol. 2007; 47; 1521
Effects of Nonsteroidal Anti-Inflammatory Drugs on Urinary Sodium Excretion, Blood
Pressure, and Other Renal Function Indicators in Elderly Subjects Consuming a
Controlled Sodium Diet:
Sodium Excretion
Hourly mean changes from baseline for ambulatory systolic blood pressure (mm Hg) on day 14
Effects of Nonsteroidal Anti-Inflammatory Drugs on Urinary Sodium
Excretion, Blood Pressure, and Other Renal Function Indicators in Elderly
Subjects Consuming a Controlled Sodium Diet
• Ambulatory systolic blood pressures were significantly higher than placebo for all treatments, with moderately greater increases for etoricoxib relative to other active treatments on day 14
• Ambulatory diastolic blood pressures were significantly higher than placebo for etoricoxib and naproxen but not for celecoxib
Does it matter?
CARDIOVASCULAR OUTCOMES
Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and
Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison
• Cyclo-oxygenase-2 (COX-2) selective inhibitors have been associated with an increased risk of thrombotic
cardiovascular events in placebo-controlled trials, but no clinical trial has been reported with the primary aim of assessing relative cardiovascular risk of these drugs compared with traditional non-steroidal antiinflammatory drugs (NSAIDs).
• The MEDAL programme was designed to provide a precise estimate of thrombotic cardiovascular events
with the COX-2 selective inhibitor etoricoxib versus the traditional NSAID diclofenac.
Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and
Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison
• 34 701 patients (24 913 with osteoarthritis and 9 787 with rheumatoid arthritis) enrolled.
• Average treatment duration was 18 months (SD 11·8).
– 320 patients in the etoricoxib group and
– 323 in the diclofenac group had thrombotic cardiovascular events, yielding event rates of 1·24 and 1·30 per 100 patient-years and a hazard ratio of 0·95 (95% CI 0·81–1·11) for etoricoxib compared with diclofenac.
• Interpretation Rates of thrombotic cardiovascular events in patients with arthritis on etoricoxib are similar to those in patients on diclofenac with longterm use of these drugs.
Incidence of thrombotic cardiovascular (CV) events in prespecified subgroups, per-protocol
Pooled data
META ANALYSES
Adverse Cardio-renal and Blood
Pressure Effects of NSAIDs
• A meta-analysis investigating the adverse cardio-renal effects of nsNSAIDs include fifty four studies with 123 NSAID treatment arms.
– The mean age: 46 years
– The vast majority (92%) of AEs were hypertension.
• The increase in mean arterial pressure was 3.59 mm Hg for indomethacin (57 treatment arms), 3.74 mm Hg for naproxen (four arms), and 0.49 mm Hg for piroxicam
– In this short-term study, NSAIDs varied considerably in their effect on blood pressure. Of the drugs studied, indomethacin and
naproxen were associated with the largest increases in blood pressure.
– The average effects of piroxicam, aspirin, ibuprofen, and sulindac were negligible [Pope et al. 1993].
Adverse Cardio-renal and Blood
Pressure Effects of NSAIDs
• A subsequent meta-analysis of 38 randomized trials and 12 randomized but not placebo-controlled trials studying the effect of nsNSAIDs on blood pressure suggested that nsNSAIDs elevated supine mean blood pressure by 5.0 mm Hg but had no effect on variables other than blood pressure.
– In this study, nsNSAIDs antagonized the antihypertensive effect of beta-blockers (blood pressure elevation, 6.2 mm Hg) more than with vasodilators and diuretics [Johnson et al. 1994].
Adverse Cardio-renal and Blood
Pressure Effects of NSAIDs
• In a single-blind, randomized, cross-over study of 29 healthy elderly subjects, given either celecoxib, 200 mg twice daily for 5 days, followed by celecoxib 400 mg twice daily for the next 5 days, or naproxen 500 mg twice daily for 10 days [Whelton et al. 2000],
– naproxen resulted in a greater decrease in glomerular filtration rate
(−5.31 mL/min per 1.73 m2) compared with celecoxib (−0.86 mL/min per 1.73 m2) after the first dose. The treatment difference became statistically significant on day 6 (−7.53 vs −1.11 mL/min per 1.73 m2 for naproxen and celecoxib, respectively; p = 0.004).
– Small, transient decreases (p < 0.05) in urinary sodium excretion were observed after the initiation of both celecoxib and naproxen treatment. The results indicated for the first time in a clinical study that cyclooxygenase 2-specific inhibition in healthy elderly subjects might spare renal hemodynamic function, although the effects on sodium excretion appeared to be similar to those of nonspecific
cyclooxygenase inhibitors such as naproxen.
Adverse Cardio-renal and Blood
Pressure Effects of NSAIDs
• The SUCCESS VI study performed a head to head comparison between celecoxib and rofecoxib with regards to their effect on blood pressure.
• This study was a 6-week, randomized, parallel-group, double blind trial in patients with osteoarthritis who were more than or equal to 65 years of age and on antihypertensive agents.
• Patients received once-daily celecoxib 200 mg or rofecoxib
25 mg.
• The primary endpoints were the development of edema, changes in systolic blood pressure, and changes in diastolic blood pressure as measured at any time point in the study.
Adverse Cardio-renal and Blood
Pressure Effects of NSAIDs
• Eight hundred and ten patients received study medication
(celecoxib, n = 411; rofecoxib, n = 399).
– Nearly twice as many rofecoxib- compared with celecoxib-treated patients experienced edema (9.5% vs 4.9%, p = 0.014).
– Systolic blood pressure increased significantly in 17% of rofecoxibtreated patients compared with 11% of celecoxib-treated patients (p =
0.032) at any study time point.
– Diastolic blood pressure increased in 2.3% of rofecoxib compared with
1.5% of celecoxib-treated patients (p = 0.44).
– At week 6, the change from baseline in mean systolic blood pressure was +2.6 mmHg for rofecoxib compared with −0.5 mmHg for celecoxib
(p = 0.007).
– This study highlighted the differences in adverse cardio-renal effects of COX-2 inhibitors partly explaining the adverse cardiovascular effects seen with rofecoxib [Whelton et al. 2001].
Who is at risk?
WHY IS THERE A DIFFERENCE?
Possible Mechanisms Associated With Variable
Cardiovascular Risk Profiles With COX-2 Inhibitors
• In principle, all COX-2 inhibitors are likely to have an adverse cardiovascular profile because the vasodilatory prostaglandin, PGI
2
(prostacyclin) is derived predominantly from the COX-2 enzyme tilting the balance towards a greater thromboxane-
A2 (TXA2) production [FitzGerald, 2003].
• However, in clinical trials, the harm signal has been noted more frequently with rofecoxib than celecoxib pointing to other mechanisms that can account for these clinically observed differences.
Possible Mechanisms Associated With Variable
Cardiovascular Risk Profiles With COX-2 Inhibitors
• Some of these differences have been explained by rofecoxib's more selective COX-2 inhibition, a longer elimination half-life and tendency to accumulate, greater oral bioavailability, and differences in pathways of hepatic metabolism [Crofford et al.
2006]
Possible Mechanisms Associated With Variable
Cardiovascular Risk Profiles With COX-2 Inhibitors
• The COX-2 enzyme, while absent in healthy vasculature, is expressed in atherosclerotic lesions [Schonbeck et al. 1999] and in transplant atherosclerosis [Baker et al. 1999].
• Strong evidence for a role of the COX-2 enzyme in atherothrombosis has been demonstrated by a reduced risk of MI and stroke in carriers of a COX-2 gene polymorphism, which reduces the expression of the COX-2 gene in plaque macrophages [Cipollone et al. 2004].
• In the diseased vasculature, COX-2 is expressed in macrophages/foam cells, medial smooth muscle cells, endothelial cells, and in the vasa vasorum, suggesting a role in plaque vulnerability [Baker et al. 1999].
Possible Mechanisms Associated With Variable
Cardiovascular Risk Profiles With COX-2 Inhibitors
• It is not clear whether COX-2 inhibition per se results in thrombogenesis.
• It has been postulated that while all COX-2 inhibitors may result in prostanoid imbalance, treatment with celecoxib in an animal study resulted in improvement in endothelial function whereas rofecoxib and diclofenac had no effect on it
[Hermann et al. 2003].
Possible Mechanisms Associated With Variable
Cardiovascular Risk Profiles With COX-2 Inhibitors
• New evidence also suggests that the harmful effects of celecoxib from PGI
2 inhibition may be counterbalanced by its beneficial effects on endothelial function, inflammation, oxidation, the L-arginine/nitric oxide (NO) and Jun N-terminal kinase pathways.
• Since NO is a powerful vasodilator and also reduces leukocyte proliferation, migration, adhesion, and vascular inflammation, these pathways are probably suffice in counteracting the TXA
2 effects [Crofford et al. 2006].
Possible Mechanisms Associated With Variable
Cardiovascular Risk Profiles With COX-2 Inhibitors
• COX-2 independent effect of celecoxib may counterbalance the adverse effect from COX-2 dependent prostanoid imbalance, accounting for neutrality of clinical effect in most clinical trials.
• Another argument underscoring the importance of the mechanisms cited above – over and above the TXA 2 and PGI 2 hypothesis – was the relative lack of protection for cardiovascular events by aspirin and clopidogrel supplementation in a valdecoxib study [Nussmeier et al. 2005].
• The attenuation of a neutral cardiovascular effect with celecoxib when used at a higher dose also supports the role of pharmacokinetics in the overall cardiovascular risk profile, as demonstrated in the APC trial
[Bertagnolli et al. 2006].
Not the End
META-ANALYSES
Cardiovascular Safety of Non-Steroidal Anti-Inflammatory
Drugs; Network Meta-Analysis
Sven Trelle; Stephan Reichenbach; Simon Wandel; Pius Hildebrand; Beatrice Tschannen; Peter M Villiger;
Matthias Egger; Peter Jüni Posted: 01/27/2011; BMJ
•
Objective: To analyse the available evidence
on cardiovascular safety of non-steroidal antiinflammatory drugs.
Data synthesis: 31 trials in 116 429 patients
with more than 115 000 patient years of follow-up were included.
– Patients were allocated to naproxen, ibuprofen, diclofenac, celecoxib, etoricoxib, rofecoxib, lumiracoxib, or placebo.
Network of comparisons included in analyses. Solid lines represent direct comparisons within randomised controlled trials. Numbers denote trials comparing corresponding interventions, with overall number of patient years of follow-up in brackets
Cardiovascular Safety of Non-Steroidal Anti-Inflammatory
Drugs; Network Meta-Analysis
Sven Trelle; Stephan Reichenbach; Simon Wandel; Pius Hildebrand; Beatrice Tschannen; Peter M Villiger;
Matthias Egger; Peter Jüni Posted: 01/27/2011; BMJ
• Compared with placebo, rofecoxib was associated with the highest
risk of myocardial infarction (rate ratio 2.12, 95% credibility interval
1.26 to 3.56), followed by lumiracoxib (2.00, 0.71 to 6.21).
• Ibuprofen was associated with the highest risk of stroke (3.36,
1.00 to 11.6), followed by diclofenac (2.86, 1.09 to 8.36).
• Etoricoxib (4.07, 1.23 to 15.7) and diclofenac (3.98, 1.48 to 12.7) were associated with the highest risk of cardiovascular death.
• Conclusions: Although uncertainty remains, little evidence exists to suggest that any of the investigated drugs are safe in cardiovascular terms. Naproxen seemed least harmful.
Cardiovascular risk needs to be taken into account when prescribing any non-steroidal anti-inflammatory drug.
Estimates of rate ratios for non-steroidal anti-inflammatory drugs compared with placebo. NSAID=non-steroidal anti-inflammatory drug; APTC=Antiplatelet Trialists' Collaboration
The END
CONCLUSION
Summary
• None of the partially selective NSAIDs such as meloxicam or nabumetone are associated with any clear safety advantage when compared with non-selective NSAIDs.
• Evidence from clinical trials suggests that the increased rate of cardiovascular events associated with coxib use is approximately three per 1000 patients per year.
• The increased cardiovascular risk attributed to coxibs is thought to be due to suppression of prostaglandin I production (endothelial lesion is COX
2 turn causes elevation of blood pressure, accelerated atherosclerosis and a heightened thrombotic response to rupture of an atherosclerotic plaque.
2 dependent) which in
Summary
• The cardiovascular safety of classical NSAIDs is unclear and may not be different to that of coxibs.
• A consistent pattern has emerged for increased cardiovascular risk for all coxibs.
• The increased cardiovascular risk extends across all NSAIDs; however the mechanism may not be related to COX
2 inhibition alone and may be due to another mechanism.
Summary
• The increased risk is independent of the use of concomitant aspirin therapy; in fact the addition of aspirin counteracts the beneficial protective effect of the coxibs on the gut.
• Selective COX
2 inhibitors are a rational choice for patients with a low cardiovascular risk who have a history of gastro-intestinal events.
• In patients who have cardiovascular disease or who are at risk of cardiovascular events, it is important to avoid NSAIDs if possible.