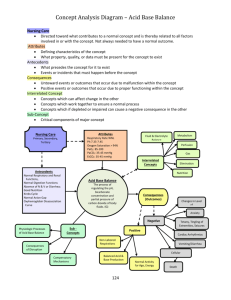
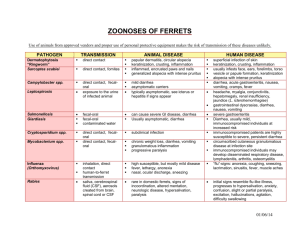
Probiotics for treatment of acute diarrhea in children: randomised
advertisement

Probiotics for treatment of acute diarrhea in children: randomized clinical trial of five different preparations Jessica Hersman M.D. January 15, 2009 Is this study applicable to my practice and my patients?… “This one time, while covering the parent pager…” Mom: “Dr.! My baby won’t stop pooping, and its really runny!” “What do I do?” Resident: “That’s too bad, unfortunately your baby probably has a viral gastro and it will just take time for the illness to run its course.” Mom: “But Dr. its everywhere!” “Do you have kids, do you know what I’m going through right now?” Resident: “No, but I have a dog :)” Mom: “Well that’s great…has you’re dog ever had diarrhea?” Resident: “No.” Mom: “Well, this is awful and I can’t take it anymore…I can’t keep him in diapers! You have to help me!” Resident: “I wish I could offer you more but just make sure that your baby stays well hydrated and come see me in clinic if if doesn’t get any better…Well, gotta run, ABP is about to close and I hear they came out with a new sandwich!… have a good day!” Acute Diarrhea Diarrhea - WHO QuickTime™ and a TIFF (U ncompressed) decompressor are needed to see t his picture. defines as passage of loose or watery stools; 3 or more per day. Among children in the U.S., diarrhea accounts for more than 1.5 million outpatient visits, 200,000 hospitalizations, and ~300 deaths per year. Probiotics? There are three general methods by which the intestinal microflora can be altered: 1. Administration of antibiotics. 2. Administration of prebiotics - dietary compounds that promote the growth and metabolic activity of beneficial bacteria. 3. Administration of probiotics or beneficial bacteria. Probiotics? - “Help me, help you!” Probiotics are microorganisims that have beneficial properties for the host. Most have been derived from food sources, especially cultured milk products. Many studies have suggested potential efficacy in several GI illnesses including IBD, Antibioitic related diarrhea, C diff colitis, and most applicable to this talk, infectious diarrhea. What’s the mechanism? The mechanism for the benefits of probiotics are incompletely understood. Mechanism of Action Suppression of growth or epithelial binding/invasion by pathogenic bacteria 1. Decrease luminal pH 2. Secrete antimicrobial peptides 3. Inhibit bacterial invasion 4. Block bacterial adhesion to epithelial cells Mechanism of Action Enhancement of intestinal barrier function. 1. Increase mucus production 2. Decrease chloride and water secretion 3. Bind epithelial cells at their apical junctions through tight junction proteins Mechanism of Action Modulation of the immune system. 1. Induce protective cytokines (IL-10 and TFG-beta) 2. Suppress proinflammatory cytokines (TNF) And now, the nitty gritty… The Objective To compare the efficacy of five probiotic preparations recommended to parents in the treatment of acute diarrhea in children. The Methods This study was a prospective single blind randomized controlled trial. The study design was discussed with six family pediatricians in three meetings. Diarrhea was defined as three or more outputs of loose or liquid stool per day. Eligible children were ages 3 to 36 months who were seen in pediatricians offices from October 1999 to September 2000 because of diarrhea. Included in the study were all children with diarrhea lasting less than 48 hours for whom parents gave informed consent. “You’re either in or you’re out…and you’re out!” Exclusion criteria Malnutrition Clinical symptoms of QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. severe dehydration Coexisting acute systemic illness Immunodeficiency Chronic disease CF Food allergy Use of probiotics in previous 3 wks Use of Abx or any antidiarrheal medications in previous 3 wks Poor compliance (<4 doses) Flow of participants through trial of probiotic preparations for treatment of childhood acute diarrhoea Canani, R. B. et al. BMJ 2007;335:340 Copyright ©2007 BMJ Publishing Group Ltd. Methods cont… All children given ORT for 3-6 hrs. Kids then fed with either full strength formula containing lactose or cowsÕmil k depending on age. Children randomized to oral rehydration alone, or Lactobacillus GG, S boulardii, Bacillus clasii, mix of L delbrueckii bulgaricus, Steptococcus thermophilus, L acidophilus, and Bifidobacterium bifidum, or E faecium strain Sf68. Parents received a coded reporting form on which to record clinical data. Parents were instructed to record daily the number of fecal ouputs and their consistency, the type and doses of probiotic preps taken by the child, the presence of vomiting and fever, any necessity for hospital amission, and all adverse events. Probiotic preparations were prescribed for five days and administered orally in 20ml of water according to the manufacturersÕinstructions. Randomization Patients were allocated to each group according to a computer generated randomization list. Random allocation was made in blocks of six to obtain groups of similar size and the sequence was concealed until treatments were assigned. The researchers responsible for enrolling the patients allocated the next available number on entry into the trial, and the parent of each child received written instructions to purchase the assigned probiotic product. The baseline features of the patients enrolled were similar with respect to age, sex, weight, and feeding regimen. Outcome Measures Primary outcome measures: Total duration of diarrhea (time in hrs from first to last abnormal stool). Number of stools/day and consistency (based on grading scale from 1-4 with 1 being normal and 4 being liquid). Secondary outcome measures: The incidence and median duration of vomiting, fever, and the number of hospital admissions in each group. Safety and tolerability were also investigated. The blind leading the blind? In order to address the problems of performing a double blind study of commercially available products the study designers used the third party blind observer method to assess efficacy. To ensure unbiased assessment, the pediatricians, who were in charge of treatment allocation, gave written instructions to parents to purchase a brand of probiotic and verified compliance on the reporting form, whereas the investigators collecting the reporting forms were blinded to the assigned treatment. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. The Results The total duration of diarrhea was significantly lower in children receiving Lactobacillus GG and in those receiving the bacterial mix than in those receiving ORT alone. 78.5 hrs and 70.0 hrs vs. 115.5 hrs respectively, both with P values of <0.001. The three other preparations had no effect on diarrhea, and the duration of diarrhea in the other groups was similar to that in the group receiving ORT alone. The Results Daily stool output was significantly lower in groups 2 and 5 (Lactobacillus GG and the probiotic mix) with P value of <0.001 starting the day after the first day of therapy. Stool consistency, judged by the scoring system 1-4, differed significantly starting the day after therapy,(P<0.001) in groups 2 and 5 versus the other groups. There was a quicker transition to more normal stool consistency in groups 2 and 5. The Results There were no significant findings in terms of secondary outcomes in any of the probiotic groups vs. those receiving ORT alone. All parents purchased the product indicated by the pediatrician and all of the preparations included in the study were well received and no adverse events were observed. The Good Is this study applicable to my patients?….Yes! Is the treatment feasible to use in my practice?…Yes! Were both statistical and clinical significance considered?…Both strains deemed efficacious in the treatment of acute diarrhea were statistically significant with P values of <0.001. I think it would have been interesting in terms of clinical significance to survey parents after completion of therapy to assess their attitudes about efficacy and satisfaction. Was there at least 80% follow up…Yes! Of the 600 patients assessed for eligibility, 29 were excluded initially for either refusal to participate or for not meeting inclusion criteria. Of the 571 patients enrolled, only 21 pts did not complete the study usually due to faster resolution of symptoms or noncompliance. (97 % follow-up) The Good…part deux Definitions - the study did a great job defining key concepts i.e. diarrhea, compliance, exclusion criteria, and expounded upon sample size in terms of what n would be necessary to obtain a certain power. Methods - the study design was clearly described and would be reproducible by a third party. The Good…Continued Randomization - Patients were allocated to each group according to a computer generated randomization list which resulted in six groups of similar size and of similar baseline characteristics. This is essential to the study design as the study enrolled 3 to 36 month old children, a patient population in which feeding regimen can very substantially among participants. For example, would the immunological effects of breast milk in exclusively breast-fed infants have had a confounding effect on the primary outcomes measured had the patients not been randomized? The Bad… Single blinded - By failing to implement a study design in which the parents, and coincidentally the data collectors/reporters are also blind to their prescribed strain of probiotic, the study designers leave their results open and subject to parental bias. In the paper the authors describe this problem as “possible confounding,” as opposed to bias, and rationalize that since these products are were not advertised in the media and because they were all available in pharmacies, parental preconceptions were unlikely. The Bad…part deux Lack of “hard facts” - Primary end points measured were not objective facts but relied upon subjective parental opinion. Grading scale of stool consistency from 1 to 4 is very open to interpretation. Data/results relied upon parental recall. The bad…continued “In conclusion…we believe that probiotic preparations should be classified as drugs…” This notion went completely unaddressed throughout the article. Safety was not mentioned in depth and though this study did speak to efficacy, whether probiotics should be regarded as food supplements or drugs in another question entirely. The bottom line Overall, I think this was a good, randomized controlled study with well thought out methods that offers an extremely relevant assessment of a potentially beneficial therapy for a ubiquitous pediatric problem. While the most glaring negative has to be the lack of a double blinded design and the reliance upon parental recall, one could argue that this deficiency allowed for a more real world setting in which to more authentically assess efficacy. Sources Canani, Roberto et al. Probiotics for treatment of acute diarrhea in children: randomized clinical trial of five different preparation. BMJ 2007; 335. Guandalini, Stefano. Acute Diarrhea in Children in Europe: Do We Know How to Treat It? Journal of Pediatric Gastroenterology and Nutrition 2008; 46: 77-80. Guandalini, Stefano. Probiotics for Children With Diarrhea: An Update. Journal of Clinical Gastroenterology 2008; 00; 000000. Guarino et al. Probiotics as prevention and treatment for diarrhea. Current Opinion in Gastroenterology 2008; 25: 1823. Sartour, R Balfour. Probiotics for gastrointestinal disease. Up to date 2008. S.C. Ng et al. Mechanisms of Action of Probiotics: Recent Advances. Inflammatory Bowel Disease 2009; 15:300-310.