Regents Unit 6: Properties of Liquids
advertisement

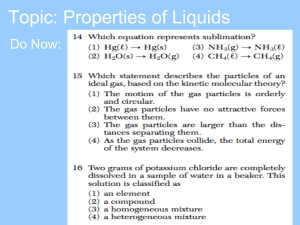
Properties of Liquids Properties of Liquids • Definite volume • Indefinite shape • Particles are close together, but can move a little bit. Liquids can flow. • Density of liquids is much greater than gases. Ex: DH2Ol is 1250X greater than DH2Og at 25C. • Liquids can be compressed but change in volume is very slight & requires enormous pressure. Viscosity • Liquids exhibit viscosity. • Viscosity = resistance to flow. • Viscosity depends on strength of intermolecular forces, sizes & shapes of molecules, and temperature. • The stronger the intermolecular forces, the higher the viscosity. • As temperature , viscosity . • As temperature , viscosity . Oil in an engine prevents direct metal to metal contact. Need a thin film of oil on bearing surfaces to prevent flaking of metal. If the oil is too thick, it won’t circulate at low temperatures. If the oil is too thin, it will lose film strength at high temperatures. Where does the marble drop fastest? water Slowest? glycerol Viscosity & Petroleum Drilling Surface Tension • Particles at the surface of a liquid exist in an unbalanced environment. No attraction from above to balance attractions from below. • Net attractive force pulling down. • Surface seeks smallest possible area. Surface Tension • Surface Tension = energy required to increase the surface area by a given amount. = measure of inward pull. • Strong intermolecular attractions High surface tension. Surfactants • Compounds that lower the surface tension of water • Disrupt hydrogen bonds between H2O molecules. • See video Capillary Action • Water forms a concave meniscus in a glass tube. • Attractive forces between water and glass > attractive forces between the water molecules. • Upward movement of a liquid in a narrow tube = capillary action. Force(H2O-glass) Force(H2O- H2O) Force(Hg-glass) Force(Hg-Hg)