homework_1_9
advertisement

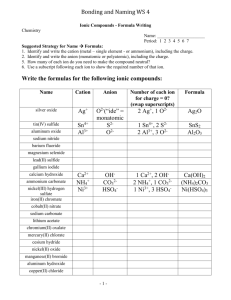
#1 Name the ions formed by these elements and as anions or cations a. selenium selenide -2 anion b. barium barium ion +2 cation phosphide ion -3 c. phosphorus anion #2How many electrons were lost or gained to form these ions? a. Fe3+ 3 lost b. O2- 2 gained c. Cu+ 1 lost #3Key concept: Explain how the charges of Group A metal and nonmetal ions are related to their positions in the periodic table. 1 Group A elements 2 3 4 5 67 The charge on the group A metals is just the group number. The charge on the non-metals is the group number minus 8 8 4 Key concept: How are the charges of some transition metal ions determined? By how many electrons they lose. This can sometimes be more than one number for a given transition metal.(example Cr2+ and Cr3+) 5 Key concept: What are the usual endings for the names of polyatomic ions? -ite or -ate examples: #6 What are the charges on ions of Group 1A, Group 3A(aluminum), and Group 5A? #7 How does a polyatomic anion differ from a monatomic anion? Chloride Cl-1 -2 Oxide O polyatomic anions have more than one Carbonate CO3-2atom, monatomic Nitrite NO2-1 anions have only one atom Hydroxide OH-1 Hydrogen sulfate HSO4 -1 Nitrate NO3-1 #8 Write the symbol for the ion of each element. Classify the ion as an anion or a cation, and name the ion. a. potassium: K+ cation; potassium ion b. oxygen: O2- anion; oxide ion c. tin (2 electrons lost): Sn2+ cation; tin (II) d. bromine Br- anion bromide ion e. beryllium: Be2+ cation; beryllium ion f. cobalt (3 electrons lost): Co3+ cation; cobalt (III) #9 Write the symbol or formula (including charges) for each of the following ions. a. ammonium ion NH41+ b. tin (II) ion Sn2+ c. chromate ion CrO42d. nitrate ion NO31-