Diffusion and Osmosis - Tana-Rae's E
advertisement

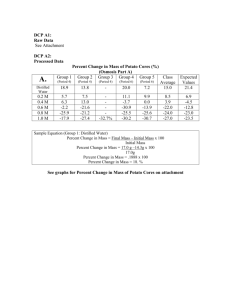
sss Diffusion and Osmosis By Lexi Parker and Tana-Rae Halfkenny Exercise 1A: Diffusion Evidence: Table 1: Diffusion Results Initial Contents Initial Solution Colour After Lugol’s and Benedict’s Final Solution Colour Presence of Glucose Positive Positive Bag: 15% Glucose and 1% Starch Milky White Light Blue Separated (clear on bottom; and milky on top) Beaker: H₂O and IKI Clear Light Yellow Foggy White Discussion: Diffusion is the movement of molecules from high concentration to low concentration. As an example, if Mr. Trentham walked into the classroom with some really stinky cologne, it would diffuse into the air and all of his students would be able to smell it because the fragrance particles dispersed into the air because the molecules will move to open spaces (low concentration). If we place dialysis tubing filled with a solution of 15% glucose and 1% starch, then the tubing will expand because there is a high concentration of water outside of the tubing (hypotonic solution) and the particles inside of the bag will diffuse out (if the selectively permeable membrane allows it). The purpose of this lab was to measure the effects of diffusion through dialysis with a solution of glucose and starch. The manipulated variable in this test is the addition of Benedict’s reagent and Lugol’s reagent, the responding variable in this lab is the colour of the substance, and the controlled variable in this test is the substances used, temperature, and the amount of reagent used. Water is the control group in this test. Water is used as the control group to show a test with no changes because it is a universal solvent. The contents in the bag reacted with the Benedict’s reagent because it has glucose in it. The bag’s final color was a result of diffusion. During the waiting period, the glucose in the bag transferred through the semi-permeable membrane into the solution. The solution was then polluted with glucose molecules causing it to be a foggy white color. The evidence that supports our answer includes the knowledge of hypotonic and hypertonic solutions. The solution in the beaker is hypotonic and the solution in the bag is hypertonic. The concentration of glucose and starch inside the bag was higher that outside the bag. This means that the glucose and starch would have passed through the semi-permeable membrane. The pores in the dialysis tubing allowed the glucose and starch to pass through, but not the Benedict’s and Lugol’s reagent. This is due to the fact that the pores are not big enough to fit the reagents. We could have measured the weight of the beaker and the bag before and after the test to get quantitative data. We could have used this data to further prove the diffusion of water in the dialysis bag. Based on our observations, the following are ranked by size: (Smallest-Largest) water molecules, IKI molecules, glucose molecules, membrane pores and starch molecules. These were determined by the reactions in the lab and information we already know. Water will obviously have the smallest pores because the atom size is very small due to the oxygen and hydrogen that make it up. Iodine and glucose both passed through the membrane so we can infer they are smaller than the membrane pores. Starch is the largest because it didn’t pass through the pores. If the results started with a glucose and IKI solution inside the bag and starch and water outside, the glucose and starch would pass through the membrane, while the starch wouldn't pass through, due to the relative sizing of the atoms. Exercise 1B: Osmosis Purpose: The purpose of this lab is to use dialysis tubing to determine solute concentration and water movement through a selectively permeable membrane by the process of osmosis. Hypothesis: If we place a dialysis bag of distilled water in a beaker of distilled water, there will be no change because there will be no net movement since this is an isotonic solution. If we place a dialysis bag of 0.8 M sucrose in a beaker of distilled water, the mass will increase because the net movement will cause the water to move into the bag, causing expansion. This will occur because the water is a hypotonic solution while the sucrose is a hypertonic solution. Variables: The manipulated variable in this lab is the addition of the dialysis bag into the distilled water. The responding variable is the mass of the bag after osmosis. The controlled variables are the amount of distilled water in the beaker, the amount of solution and the time waited for osmosis to occur. Evidence: Table 2 : Dialysis Bag Results : Individual Data Contents in Bag Initial Mass Final Mass Mass Difference Percent Change in Mass Distilled Water 23.55 22.49 1.06 4.50% 0.8 M sucrose 20.05 23.45 -3.4 16.96% Table 3 : Dialysis Bag Results : Class Data Grp. 1 Distilled Water Grp. 2 0% Grp. 4 Grp. 5 1.4% 0.2 M 0.4 M Grp. 3 5.9% Grp. 7 Total Class Avrg. 4 1.3% 4.6% 12.6 4.2% 5.4% 22.3 7.4% Grp. 8 2.6% 4% 11% Grp. 6 4% 0.6 M 10% 11% 0.8 M 13.6% 1.0 M 14.7% 𝑃𝑒𝑟𝑐𝑒𝑛𝑡 𝐶ℎ𝑎𝑛𝑔𝑒 𝑖𝑛 𝑀𝑎𝑠𝑠 = = 16% 23% 25.4% 21 10.5% 52.6 17.5% 40.1 20.1% 𝐹𝑖𝑛𝑎𝑙 𝑀𝑎𝑠𝑠 − 𝐼𝑛𝑖𝑡𝑖𝑎𝑙 𝑀𝑎𝑠𝑠 ∗ 100 𝐼𝑛𝑖𝑡𝑖𝑎𝑙 𝑀𝑎𝑠𝑠 18𝑔 − 20𝑔 ∗ 100 20𝑔 = −0.1 ∗ 100 = −10 The sucrose solution in the beaker would have been hypertonic to the distilled water in the bag. Graph 1: Dialysis Bag Results Exercise 1C: Water Potential Purpose: To determine what will happen when a potato is placed in solutions with different solute potential. Hypothesis: If we place a potato in distilled water, it will cause it to expand because the water potential is lower in the potato which causes the water to flow into it because water will always move to an area of lower water potential. If we place a potato in 0.8 M sucrose, it will cause it to shrivel up because the water potential in the sucrose is lower which causes the water to flow into it because water will always move to an area of lower water potential. Variables: The manipulated variable in this lab is the placement of the potatoes in the solutions. The responding variable is the reaction of the potato in the solutions. The controlled variables are the amount of potatoes used in each solution, the amount of solution, and the amount of time waited for the reaction. Evidence: Table 4: Potato Core: Class Results Contents Initial in Mass Beaker Distilled 3.32 Water Final Mass 3.88 Mass Percent Difference Change in Mass -0.56 16.87% 0.2 M 5.00 5.87 0.87 17.00% 0.4 M 2.20 2.26 -0.60 -2.70% 0.6 M 2.10 1.87 0.23 10.90% 0.8 M 2.67 2.06 0.61 -0.23% 1.0 M 5.27 6.45 1.18 22.40% Exercise 1D: Calculations of Water Potential from Experimental Data