view the UCKRN powerpoint presentation on CSP
advertisement

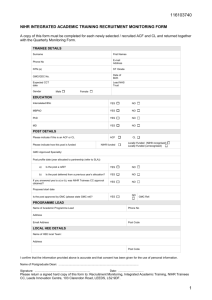
National Institute for Health Research Coordinated System for gaining NHS Permission (NIHR CSP) A single system for processing and reviewing applications for NHS permission The Challenge Research & Development approval and set up times are a big factor affecting the UK’s competitiveness in conducting clinical studies. Who is leading the development of NIHR CSP ? NIHR Clinical Research Network Coordinating Centre (CRN CC) In close collaboration with key stakeholders including: UK Clinical Research Collaboration National Research Ethics Service NHS Research & Development Forum Department of Health Industry NIHR Clinical Research Networks Researchers What is the NIHR Coordinated System For gaining NHS Permission (NIHR CSP)? A consistent, quality assured and standardised process for gaining NHS permission to conduct portfolio research in England A single application point for NHS permission, through the Integrated Research Application System (IRAS), for multi-site and single site studies A system managed by a national CSP Unit and the Comprehensive Local Research Networks, to ensure a coordinated approach with local input Why do we need NIHR CSP? The benefits of the new system are: Consistency A consistent and comprehensive set of governance checks Speed To streamline processes to reduce NHS R&D approval times Predictability A single system for processing and reviewing applications for NHS permission. Speed Association for British Pharmaceutical Industry (ABPI) metrics for 2008 show: 82 day MEDIAN time from R&D notification to sign off 187 day MEDIAN time from first submission to first patient / visit Slow start up leads to reduced recruitment periods for globally competitive studies Benefits Single application point through IRAS* for all NHS sites. A standardised, coordinated approach resulting in more rapid NHS permission for sites Single, secure online databases and document repository Ensures that specific checks are only conducted once for multi-centre studies, with clear distinction between local and national checks Built upon best practice in research governance already in place within the NHS How do I apply to NIHR CSP? Researchers will access NIHR CSP through the Integrated Research Application System (IRAS) A single application point for all NHS permission NIHR CSP will be available for all studies which are automatically eligible or adopted into the NIHR portfolio NIHR CSP Support People & Training NIHR CSP is coordinated by a national NIHR CSP Unit National CSP Unit work in collaboration with the 25 CLRNs, who employ R&D staff within their local NHS organisations Information Systems A web-based system (CSP ReDA) with electronic document repository will facilitate NIHR CSP processing between the national CSP Unit and CLRNs Management NIHR Network Coordinating Centre work with key partners to ensure effective governance NIHR CSP in Practice NIHR CSP will be conducted in accordance with national Standard Operating Procedures (SOP) These procedures will clearly define which governance checks are global (undertaken once per study), which are local (undertaken at every participating site) and who is responsible for carrying them out Quality assured process enables predictability i.e. common approach NIHR CSP will be compatible with similar systems being developed in Northern Ireland, Scotland and Wales Testing NIHR CSP First stage pilot - Complete Mapped the approval timelines for ten existing multi-site studies to identify any limiting steps and look at the critical permissions pathway Looked at a new study currently going through approval to identify how to speed the permissions process up Used the new system to facilitate permission for a number of studies Testing NIHR CSP Second stage pilot - Complete Focused on how studies progress through CSP and the CSP software (ReDA) Focused on the CSP Operating Guidelines and the CSP software (ReDA) Gained feedback from active researchers on the NIHR CSP user interface Gained feedback from CLRN RM&G managers who have responsibility to manage the process NIHR CSP Go-Live NIHR CSP is on schedule for go-live on 18 November 2008 and will be continuously developed Initially available to studies within the NIHR Clinical Research Portfolio Once all checks complete there is a built in double check called CSP sign off – 7 day target The timeline from CSP sign off to signed NHS permission letter is 21 days Conclusion CSP will provide national clarity to the process of gaining NHS permissions CSP will benefit NIHR key stakeholders CSP is a system step change and will take some time to function properly CSP is a major step towards busting bureaucracy Visit the website for further information http://csp.ukcrn.org.uk Or contact the CSPU team at: csp@ukcrn.org.uk For NIHR Portal users visit http://portal.nihr.ac.uk/sites/ukcrn/CSPint For non NIHR Portal users visit http://portal.nihr.ac.uk/Pages/Abouttheportal.aspx For IRAS visit https://www.myresearchproject.org.uk/ Faculty NIHR Key Workstrands Investigators and Senior Investigators Trainees Associates Research Universities Infrastructure NHS Trusts Clinical Research Networks Research Projects and Programmes Patients and Public Clinical Research Facilities and Centres Research Units and Schools Research Governance Systems Research Information Systems Systems