NASA CEA Tutorial: Chemical Equilibrium for Rocket Propulsion
advertisement
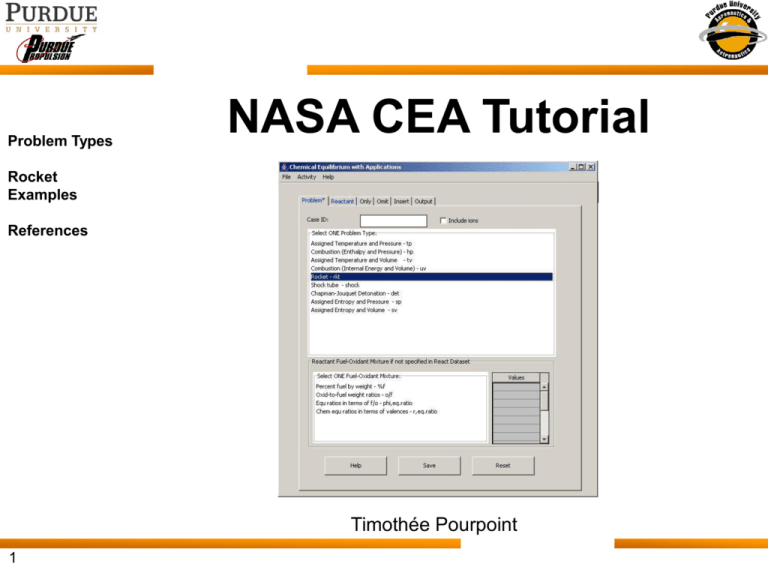
Problem Types NASA CEA Tutorial Rocket Examples References Timothée Pourpoint 1 Online Access & Documentation http://www.grc.nasa.gov/WWW/CEAWeb/ceaWhat.htm CEA: • calculates chemical equilibrium product concentrations • determines thermodynamic & transport properties 2 supporting documents: Request a download 2/15 1. Detailed analyses of chemical equilibrium calculations 2. User manual and program description Installation Overview Download desired version (Windows) CEA GUI Download consists of the following files: 1) CEAgui-jar.zip 2) CEA+Fortran.zip 3) CEAexec-win.zip Notes from: Readme_CEAgui-win.txt If your local system has NOT installed the Java2 SDK (J2SDK), you must install the Java 2 Runtime Environment (JRE) which consists of Java Virtual Machine, the Java platform core classes, and supporting files. It is the runtime part of the J2SDK. j2re1_4_1_03-windows-i586.exe can be downloaded from "http://java.sun.com/j2se/1.4/" for Java2 SDK from Sun Microsoft Inc. The CEAexec Package installation procedures for Windows: 1. Create the installation Directory on your local machine (e.g. mkdir C:\CEAexec) 2. Download all three ZIP files and the executable file into the SAME directory (e.g. cd C:\CEAexec) 3. Unzip the Fortran Source Code (CEA+Fortran.zip) and save into the SAME directory. 4. Unzip the CEA GUI Execution file(CEAexec-win.zip)and save into the SAME directory. 5. Unzip the CEAgui JAR file (CEAgui-jar.zip) and save into the SAME directory. 6. Double-click on the file (j2re1_4_1_03-windows-i586) to install Java Runtime Environment (JRE) by using the system DEFAULT directory (e.g. C:\Program Files\Java Soft\JRE\1.4) 7.Execute the batch file (CEAexec-win.bat) for using the file CEAgui.jar. 3/15 Problem Types (1/2) Assigned Temperature and Pressure – tp Chemical equilibrium composition and properties will be calculated for selected temperatures and pressures. Combustion (Enthalpy and Pressure) – hp Enthalpy held constant resulting in an adiabatic flame temperature, equilibrium mixture properties and composition. Assigned temperatures and volumes – tv Chemical equilibrium composition and properties are calculated for assigned temperatures and a set of either assigned specific volumes or densities. Combustion (Internal energy and volumes) – uv Chemical equilibrium composition and properties are calculated for assigned internal energies and assigned total reactant specific volumes or densities. Rocket – rkt Theoretical rocket performance parameters can be calculated for: infinite-area or finite-area combustors chemical equilibrium for all points or freeze composition after combustion, throat, or any exit point. thermal transport properties 4/15 Problem Types (2/2) Shock tube – shock Calculates shock properties in terms of assigned velocities. Chapman-Jouquet detonation – det Chapman-Jouguet detonation properties are calculated for a set of unburned gaseous reactants at assigned temperatures and pressures. Assigned entropy and pressures – sp Converges on both the gaseous and condensed products for a reactant mixture with an assigned entropy and set of pressures. Assigned entropy and either specific volumes or densities – sv Chemical equilibrium composition and properties calculated for assigned entropy and each assigned total reactant specific volume or density. 5/15 Rocket Problems INFINITE-AREA COMBUSTOR All points are isentropic with respect to the combustion point. Can assume chemical equilibrium or products frozen after a specified point. Freeze points selections are combustion, throat, or exit1. FINITE-AREA COMBUSTOR In this chamber combustion is a non-isentropic, irreversible process. During the burning process, part of the energy released is used to raise the entropy, and the pressure drops. Expansion in the nozzle is assumed to be isentropic. Can only assume chemical equilibrium. 6/15 Example Continue with our example from class: Oxidizer: O2(l) Fuel: CH4(l) Pc = 1000 psi O/F = 4.0 (near stoichiometric) Ac/At = 3.0 Ae/At = 10.0, 25.0, 50.0 7/15 Inputs to Example (1/4) Problem Tab Click Notes: 1. Make sure all inputs are validated by clicking outside of input cells 2. Make sure there is no space before an input 8/15 Inputs to Example (2/4) Reactant Tab Reactant Selector Notes: 1. Propellants can be in gaseous, condensed (solid/liquid), or atomic form 2. Use the search function to quickly find desired propellant 9/15 Inputs to Example (3/4) Output Tab Thermal transport properties are very useful for calculations related to cooling requirements in chamber or at nozzle throat. Parameters typically used in Bartz equation (Ref. 3): Output properties from CEA include: • Viscosity [millipoise] • Specific heat, [kJ/kg-K] • Thermal conductivity, [mW/cm-K] • Prandtl number 10/15 Inputs to Example (4/4) Save input file (File, Save) View input file Click on “Activity” Click on “View Current INPUT file Ctrl+I” RUN CEA2 11/15 Output from Example (1/3) Equilibrium 12/15 Frozen Output from Example (2/3) Equilibrium 13/15 Frozen Output from Example (3/3) Equilibrium 14/15 Frozen References Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications I. Analysis, Sanford Gordon and Bonnie J. McBride, National Aeronautics and Space Administration - Lewis Research Center, NASA RP-1311 Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications II. User's Manual and Program Description, Bonnie J. McBride and Sanford Gordon, National Aeronautics and Space Administration - Lewis Research Center, NASA RP-1311-P2 Modern Engineering for Design of Liquid-Propellant Rocket Engines, Dieter K. Huzel and David H. Huang, Progress in Astronautics and Aeronautics, Volume 147, AIAA 15/15






