What is informed Consent?
advertisement

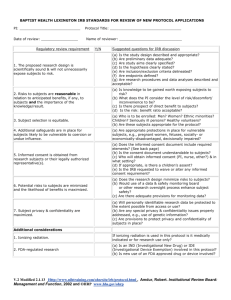
Identify consent process requirements Distinguish between IRB, PI/Designee consent process responsibilities Identify with what went wrong? Summarize tips to avoid deficiencies "A word is not a crystal, transparent and unchanged; it is the skin of a living thought and may vary greatly in color and content according to the circumstances and the time in which it is used" — Oliver Wendell Holmes Jr. DHHS – 45 CFR Part 46.116 Common Rule FDA 21 CFR 50.25 Part 50 (Informed Consent) Part 56 (IRB) ICH GCP E-6 Section 4.8.10 “No investigator may involve a human being as a subject in research… unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence. The information that is given to the subject or the representative shall be in language understandable to the subject or the representative” 45 CFR 46.11 A document that provides a summary of the research and explains the subjects rights as a participant It is designed to outline and be a reference regarding what is expected of the participant information exchange including subject recruitment materials, verbal instructions, written materials, questions and answer sessions and signature documenting consent with date. Protecting study volunteers in research Cynthia McGuire Dunn, MD & Gary Chadwick, PHARM.D, mph 1999. Disclosure of relevant information to prospective subjects about the research; their comprehension of the information, and their voluntary agreement, free of coercion and undue influence, to research participation. http://ohsr.od.nih.gov/info/sheet6.html Detailed description of the method for obtaining informed consent Who Where The process submitted for IRB approval Changes in the process are submitted as amendments PI assures the informed consent process in research is an ongoing exchange of information throughout the course of the research and it is documented Oral Telephonic E consent Video Facsimile The person must be trained regarding informed consent process and be knowledgeable about study FDA Requirements: IRB must know who will conduct consent process FDA does not require the that the PI personally conduct the consent process. ECOG Requirements: “Legally, it is the physician’s responsibility to discuss the study with the patient and obtain the written consent.” “After an initial discussion it may be the physician, nurse, or CRA who provides further details to the patient.” 7.2.6 “Presenting the Consent Form to the Patient,” ECOG Protocol Management 41% Study nurse 21% PI 19% No one 12% Family member 8% Other Source: 2002 Center Watch Survey of 1,561 Study Volunteers Fetuses, Pregnant Women, and Human In Vitro Fertilization Prisoners Children Elderly Cognitively Impaired Minorities Etc. Cognition/capacity Level of education Social/cultural values Language Age Environment Anxiety/fear Pain Influence of medications Quality of disclosed information Readability of informed consent “the belief that the purpose of a clinical trial is to benefit the individual patient rather than to gather data for the purpose of contributing to scientific knowledge” The subject believes that his medical needs will determine his assignment to a treatment group or the PI will modify the protocol to serve his own medical need. The subject has unreasonable expectations about the likelihood of benefit from study participation. In this example the subject believes the PI will not administer treatment that might harm them, but rather, will provide interventions that only help them. When the study is closed and final reports are issued At each interaction, the investigator must reassure Voluntary participation continues New information is given to the subject FDA Mandates ICH/GCP 4.8 suggests FDA has no regulations concerning delegation of consenting although it is discussed in the FDA Information Sheets FDA only requires that a copy of consent be provided to subject consent is obtained the same day that the subject's involvement in the study begins, the subject's medical records/case report form should document that consent was obtained prior to participation ICH allows the delegation of the informed consent process to a designee ICH recommends the person conducting the informed consent process sign and date the consent form ICH recommends that the subject receive a signed and dated copy of the consent form If research“FDA Consent information sheet” FDA and ICH BOTH require the IRB to review: The informed consent, process, protocol, advertisements, and the Investigator's Brochure ICH/GCP 3.1 also recommends IRB review of: Subject recruitment procedures Written information provided to subjects Information about subject compensation Investigator's current CV and/or other documents evidencing qualifications DHHS OHRP FDA Other Federal Agencies (NIH, CDC and CMS State Law Institution IRB Policy Policy IRB Policy Department Policy Research Team SOP’s Study Protocol/ Contract Depending on funding and/or Dept policy ICH/GCP Emergency, life threatening situation that requires intervention Minimal risk study with IRB approval Ultimate protector of the subject’s rights and safety Be personally certain that each subject is adequately informed and freely consents to participate in the investigator’s research Assure that every reasonable precaution is taken to reduce risk to a minimum for the subject The investigator is responsible for whom he delegates authority Follow the protocol and IRB approved study documents Obtain consent before initiating ANY studyspecific procedures Provide a quiet, comfortable, and private setting Explain the consent procedures and process to the subject Ensure sufficient time to consider all options Access the subject's reading abilities, cognitive status now and throughout study Access subjects understanding Ensure the subject is the one who wants to participate, free from coercion or other undue influence Consistent with IRB approved process Provide additional safeguards as required Provide new information promptly Provide a copy of the consent document and each revised consent document to the subject During the interview be aware of the things you might be saying that would affect voluntariness or be coercive “free drug” “free treatment” “I think this is best for you” “do this for me” Document process and response from patient Know the protocol Introduce yourself and state who referred you Do not depend on subject enrolling You are not a salesman Provided consent document for review Methods of conveying information differs Check list Read consent to subject Read highlights /review calendar Video Electronic Web-based resources Establish a relationship with the subject Provide privacy Assess views on research vs. standard of care Keep the subject in the center of the process Be an active listener Ask open-ended questions (test back) Be aware of non-verbal messages Empathize with the subject’s concerns Be a teacher by educating the subject and verifying his understanding of the research study Assure withdrawal is possible at ANY time Inform other options are available Be available anytime for any question Do not rush the process or the subject Informed Consent Worksheet Date of Consent:__________________ Name of Study:_____________________________ IRB Study Number:__________________________ Patient Name:_____________________ Patient MRN:_________________ _Study ID#:__________ The following has been explained to the potential study subject, and the subject has been offered the opportunity to ask questions regarding the study: TOPIC COMMENTS Purpose of the study _________________________________ Qualifications to participate _________________________________ Location and participants _________________________________ What will happen during the study _________________________________ Risk and benefits _________________________________ Study related injury or illness _________________________________ Alternative treatments _________________________________ Confidentiality _________________________________ Study costs _________________________________ Compensation _________________________________ Who to contact with questions _________________________________ Voluntary participation _________________________________ Termination of participation _________________________________ Questions or comments: __________________________________________________________________ __________________________________________________________________ Does the patient state an understanding of the study and procedures and agree to participate? ___yes ___no _____________________________________ Person administering consent Signed copy given to patient? ___yes ___no __________________________ Date / Time Copy in patients chart? ___yes ___no Ask if problems arose since last visit (A/E’s) Provide new information if applicable Encourage questions each visit Talk about what comes next Re-assess subjects desire to continue each visit Assess compliance (diary, meds etc) If subject regains cognitive ability New information becomes available Significant protocol changes New surrogate is identified IRB instructs you to re-consent Investigator has the option to re-consent for longitudinal data collection time points Non compliance Complaints to the IRB, the institution, the OHRP or the FDA Ongoing Interactive process Different for every subject Different for every study Essential for study success IRB approved Providing clear definition between where standard of care leaves off and research begins Allows re-education Requires re assessment of subject understanding with each visit Method of presentation appropriate for type of study and population Voluntary participation Environment Length of time devoted to the process Adequate time offered to ask questions How subject demonstrates understand of the study and desire to participate Promptness of reporting new information Auditing/Compliance Reviews If SOP’s exist are they followed Confirm consent process IRB approved Change implemented only after IRB approval Consent signed prior to ANY procedures Consenting person has appropriate training Consenting person listed as KSP You know you want to: Volunteer to have your consenting process observed Contact Wendy Lloyd by phone (936-7106) or by email (wendy.lloyd@vanderbilt.edu) in advance or just prior to consenting Signatures of subject and consenting person on different dates Consent and study procedures on same date Consent was performed by an untrained or unqualified care provider Person consenting is not listed as KSP Unable to locate consent for subject on study Subjects not re-consented with revised consent as instructed Multiple consent documents for same patient with no explanation why Person consenting did not state the purpose or procedures of the study Consent document left on clip board for subjects to complete and return to nurse if interested Person consenting the subject did not sign the form Only use approved consent process Confirm all personnel consenting subjects are KSP Document training and qualifications of all KSP Confirm person consenting knows the protocol Conduct random audits of the consent process documentation ( if form created, confirm use) Review the FDA Warning Letters and FDA IRB Information Sheets –“A Guide to Informed Consent” Become familiar with the Regulations, state law, institutional and IRB Policies Call the VHRPP Know the protocol Only do what you are trained to do Volunteer for consent observation Ask subjects if they felt fully informed Seek education Stay organized If you find something don’t hide it Network Become certified If you don’t know ask Although the regulations place the burden of responsibility on the PI, the protection of human research subjects is a shared responsibility among all research professionals involved in the conduct of the study Members of a research team have a moral obligation to uphold the ethical and regulatory standards by which human subjects research is conducted Measure the immediate level of patient comprehension at the time surgical consent is obtained and the effect of time on this level of understanding. 100 subjects consented When test administered Percent of Correct answers 98 Preoperatively 70.70 75 1st Post op (no more than 2 weeks) 59.50 39 1st Post op (no more than 2 weeks) 60.8 In addition, the effect of sex (no difference), education level (college scored better), and age (<50 scored higher) on comprehension. http://www.ncbi.nlm.nih.gov/pubmed/22005875 2011 study Crepeau AE, McKinney 18 year old with partial ornithine transcarbamylase deficiency Usually fatal in infancy The first person to die from gene therapy FDA suspended all gene therapy trials and other experiments Hearings on quality of oversight and safety President Clinton demanded improvements in consent and access to information about gene therapy research Gelsinger v. Univ. of Penn. (C.P. Phila. Co., 2000) 18 year old boy died during a gene transfer experiment for ornithine transcarba – mylase deficiency. PI founded company that sponsored trial – owned 30% of stock University of Pennsylvania owned 5% of stock University’s Conflicts of Interest Committee was aware of the potential conflict of interest between the parties – allowed study to proceed Gelsinger v. Univ. of Penn. One of the many counts Intentional Assault and Battery Informed consent process did not disclose Monkey deaths Previous adverse events Relationship between PI and sponsor True efficacy results Confidential settlement Sued University trustees, PI, sponsor and hospital Each interaction is different because every subjects, circumstance, question, communication style is different. It is up to each one of us to take the consent process serious and fully inform each subject By possibly increasing subject recruitment and retention on a wide scale Playing a substantial role in shaping public perceptions of the value of clinical research For federal guidance on obtaining informed consent of human research subjects, see the following websites: General requirements for informed consent http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.20 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.23 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.24 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.25 Documentation of informed consent http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.27 Research involving pregnant women, fetuses or neonates http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.204 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.205 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.206 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.207 Research involving children (also found in 21 CFR 50.50-56) http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.404 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.405 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.406 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.407 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.408 http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#46.409 Pediatric research “assent”decision matrix http://www.hhs.gov/ohrp/panels/407-01pnl/riskcat.htm Office for Human Research Protections (OHRP) informed consent tips http://www.hhs.gov/ohrp/policy/index.html OHRP informed consent FAQ http://answers.hhs.gov/ohrp/categories/1566 Vanderbilt IRB Policy http://mcapps01.mc.vanderbilt.edu/IRB/policy&procedures.nsf/(WebTa bleOfContents)/8AF759048966C29D86257731005ECD1F?OpenDocument If you have additional comments or questions feel free to contact me Wendy Lloyd Wendy.lloyd@vanderbilt.edu 615-936-7106