What is Wolbachia?
advertisement

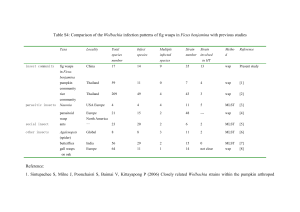
Wolbachia pipientis , an agent of speciation? "So, naturalists observe, a flea / Hath smaller fleas that on him prey / And these have smaller fleas to bite 'em / And so proceed ad infinitum.“ Jonathan Swift, of Gulliver's Travels Made by Robin Groch What is Wolbachia? • Obligate endosymbiont bacteria – a genus of the eubacteria domain • Wolbachia is an α-proteobacteria – descended from gram-negative bacteria • Related to mitochondria – also a gram-negative, fused to ancestor eukaryote 2 billion years ago • Symbiont of Ecdyzoa (arthropods) and nematodes • Cytoplasmically inherited rickettsiae that are found in reproductive tissues (ovaries and testes) Tree of Life Alpha-Proteobacteria Wolbachia Wolbachia lost the ability to cause disease in vertebrates includes nitrogen-fixing bacteria, chemoautotrophs, and chemoheterotrophs. Members of this sub-phylum grow at low-nutrient levels (oligotrophic) and some have stalks(prosthecae). Distribution of Wolbachia • No vertebrates (humans included) are known to carry Wolbachia • infection is rampant in the invertebrate world (est. ~ 20 million species) – – – – • Estimates range from 15-20% of all insect species, 154 species so far 17 isopods and freshwater shrimp Arachnids (mites and spiders) Parasitic nematodes Closest relative Wolbachia are a group of rickettsiae – Ehrlichia equii, Ehrlichia canis, Cowdria ruminata, and Anaplasma marginale. – These are blood parasites of mammals that are vectored by arthropods • Bacteria in the genus Rickettsia are still more distantly related – genus includes several arthropod-vectored disease agents, including the causative agents of Rocky Mountain spotted fever, murine typhus, and scrub typhus, as well as a cytoplasmically inherited male-killing bacterium found in ladybird beetles Some of the Hosts of Wolbachia Achaera encedon Drosophilia Parasitic nematode Nasonia Armadillidium vulgare Formica exsecta Adalia bipunctata Wolbachia Strains and their hosts Nematodes Arthropods 100 Mya D* C* B (isopods and insects) A (insects) ?? Mya 58-67 Mya 600 mya 100 Mya, proposed time of endosymbiosis of Wolbachia *Strains C & D are found in filarial nematodes Proposed horizontal transmission from arthropods to nematodes 100 mya What is Wolbachia? • Diversity of Behaviors – Parasitism • Manipulation of host reproduction in which one organism (the parasite) lives in or on the body of another (the host) and obtains nutrition and other benefit from it. A parasitic relationship is usually to the detriment of the host but some host species have evolved remarkable tolerance to high levels of parasitic infection – Mutualism • Wolbachia Hypothesized to help host metabolism, host benefits Wolbachia has a home and a method of infectious transmission – Commensalism • loose association in which Wolbachia benefits with no apparent advantage or disadvantage Note: The boundaries between these three kinds of symbiosis are not always clear because it can be difficult to establish advantage or disadvantage in the relationship. Filarial Worms: Mutualism Example Filarial worms (nematodes are associated with Elephantiasis and Riverblindness diseases. Wolbachia are parasites in most invertebrates, researchers suspect that they live mutualistically with nematodes. Perhaps the clearest sign that the worms derive some benefit from an infection is the fact that they suffer if their Wolbachia are wiped out by antibiotics. Onchocerca ochengi, a filarial nematode in cattle, for example, die when their bacteria are destroyed. In other species, the females simply become sterile Wolbachia and skewing the host’s sex-ratio Alteration of timing of host’s reproductive cycle results in a variety of reproductive phenotypes : • Parthenogenesis (Infected virgins produce only daughters) • Male Killing (male embryos die, female embryos develop into infected females) – Hymenoptera – Acraea encedon (Ugandan butterfly) – Adelia bipunctata (two-spot ladybird) • Feminization (Infected genetic males reproduce as females) • Cytoplasmic Incompatibility (infected male and uninfected female yield no viable embryos) – Armadillidium vulagare (wood louse) – Most common mechanism Parthenogenesis Induction •Infected virgins produce only daughters, score one for the symbiont • Appears to be restricted to Hymenoptera (wasps) • evidence of over 40 species affected •Hymenoptera have particular sex determination “arrhenotoky” (males are haploid, females diploid) •Reversed with antibiotics •Best case scenario for bacteria •No superfluous males •Mechanism •Takes place in infected females unfertilized eggs •1st mitotic division aborted in anaphase •Yields 2N in unfertilized egg, therefore female •Costs and Benefits for the host •Infected females produce less offspring •If bacteria completely successful, asexual reproduction results (bad for both) •Older females harbor less Wolbachia (have laid more eggs) •Older females produce more males. Male Killing or “Dead Man Walking” • Maternal inherited factors- kill male progeny during embryogenesis • High sex-ratio of females • In Acraea encedon, 80-90 % population female • Wolbachia very adaptable – Male killing in male heterogametic Adalia – Male killing in female heterogametic Acraea • Can reverse with antibiotics Acraea encendana and leks Sexist microbe. Wolbachia favor females, like this Ugandan butterfly, because they will carry on the lineage. CREDITS: COURTESY OF BACCHI AND BANDI; FRANCIS JIGGINS/CAMBRIDGE UNIVERSITY Let’s Lek • Sex-ratio shift towards females, bacteria kills off males • Now the males are choosy, a valuable commodity • Female role reversed, form dense swarms (leks) (350 butterflies/200m²) • Males prefer uninfected females, but mistakes occur (which if fine with Wolbachia) Feminization • In Armadillidium individuals become female unless androgenic gland present – Gland produces androgenic hormones to promote male sex determination • When Wolbachia is inherited from female a suppression factor prevents the formation of the gland – converting males into reproductively competent females (although intersexes can also produced). • • • Symbiont wins, all female offspring Yum, Adalia eats unhatched males Curable with antibiotics Cytoplasmic Incompatibility (CI) • Wolbachia-induced CI is a reproductive incompatibility between sperm and egg, – which typically results in zygotic death in diploid species or male production in haplodiploid species – The bacteria are transmitted in eggs but are not transmitted through sperm • CI takes two forms, unidirectional and bidirectional. – Unidirectional incompatibility typically occurs when the sperm from a Wolbachia-infected male fertilizes an uninfected – The reciprocal cross (uninfected male and infected female) is compatible. – Bidirectional incompatibility typically occurs when a male and a female harbor different strains of Wolbachia that are mutually incompatible egg. CI Outcomes Paternal Chromosome eliminated embryo become haploid Diploid organism Haplodiploid organism Embryo dies CI mortality If a wasp, Normal male Male-biased Sex-ratio Some mites, embryo Dies, CI mortality What’s Happening? • Uninfected organisms – Sperm enters egg – Sperm chromatin decondenses, forms paternal pronucleus – Paternal histones removed and replaced by female histones – Replication commences, chromosomes condense for mitosis – Paternal and maternal pronuclei fuse to make diploid nucleus of zygote • Infected organisms – Only female pronucleus forms and undergoes first cleavage – Paternal pronucleus does not condense into individual chromosomes – Paternal chromosomes appear as diffuse tangle of chromatin and gets fragmented during 1st mitotic division – If 2N organism, then embryo is haploid, & either fails to develop or female sterile or has reduced fecundity – Post-zygotic barrier (aneuploidy or failure of syngamy) Modification-Rescue Model of CI • • Incompatibility apparently involves a two component system bacterial "modification" of sperm and a bacterial "rescue" in the fertilized egg. – bacteria present in the testes modify the developing sperm (possibly via chromatin binding proteins). – The same bacterial strain must then be present in the egg to rescue this modification. If rescue does not occur, then incompatibility between the egg and sperm results. – consistent with unidirectional incompatibility (modified sperm from infected males are not rescued by uninfected eggs) and bidirectional incompatibility (different bacterial strains use somewhat different modification-rescue systems). • Two general biochemical models have been proposed, either – (a) Wolbachia in the male produce a product that disrupts sperm processing in the egg (unless rescued) or – (b) bacteria in the male act as a "sink" to bind away a product necessary for normal processing of the sperm in the egg – consistent with the "sink" hypothesis, a number of host chromatin-binding proteins (such as H1 histone-like–protein) have been found to bind to Wolbachia within host cells Nasonia Timing Is Everything for Wolbachia Hosts Carl Zimmer (Science 296, 999-1000 (2002) Cytoplasmic Incompatibility in Nasonia “ A case of bad timing” • Latest model: – Asynchrony between maternal and paternal pronuclei chromosomes during 1st mitosis – Cause: CdK1/cyclin B and disruption of cell cycle checkpoints – Wolbachia inhibiting cell cycle timing in unidirectional CI • Infected sperm modified, no rescue in eggs • Rescue = female pronucleus delayed also “ A case of bad timing” continued • Why do reciprocal matings work? – Infected female have live Wolbachia which can change the timing of the uninfected sperm • Why don’t bidirectional matings work? – Males and females infected with different strains that can’t align the timing of mitosis, asynchrony still occurs… Figure 1 Wolbachia and cytoplasmic incompatibility. Cytoplasmic incompatibility means that when a male host infected with Wolbachia (W+) mates with an uninfected female (W- ), no offspring are produced. All other matings are fully compatible and result in the production of offspring. The consequence of this system is that the maternally transmitted Wolbachia tend to spread through the host species. Nature 409, 675 - 677 (2001) © Macmillan Publishers Ltd. Evolution: Infectious speciation MICHAEL J. WADE A New Twist • Restoration of fertility in Sex-lethal Drosophilia • infection with the parasite makes a bad fly mutation more benign - but at the cost of potentially addicting the species to the parasite • Wolbachia probably produce some protein that interacts with the protein encoded by the sexlethal gene. The bacteria apparently do not affect expression of the gene or bypass the protein in the pathway leading to egg production Wolbachia rescues oogenesis defects b, c, Ovaries from 4-day-old Wolbachia-infected (b) or uninfected (c) Sxlf4/Sxlf4 females. Magnification 12 A host–parasite interaction rescues Drosophila oogenesis defects DIANA J. STARR AND THOMAS W. CLINE . Nature 418, 76 - 79 (2002) Infectious Speciation • Wolbachia are known to alter early development and mitotic processes in their hosts • Wolbachia have implications for important evolutionary processes. Of particular interest is their potential role as a mechanism for rapid speciation • Wolbachia may promote rapid speciation by causing reproductive incompatibility between populations especially when bidirectional incompatibility occurs. Infectious Speciation (2) • Post-zygotic barriers that are not germ-line generated and reversible via antibiotics • Quicker route to speciation • Need earlier level of speciation… host speciation Figure 2 Genetic and infectious models of speciation. a, A standard genetic model in which the initial state is an ancestral population of a species that is homozygous at both of two gene loci b, Infectious speciation, which parallels the genetic model. Reciprocal cytoplasmic incompatibility between WA males and WB females, and WB males and WA females, prevents hybridization, so in effect the daughter populations are new species even though they remain genetically identical to one another and to the ancestor. Nature 409, 675 - 677 (2001) © Macmillan Publishers Ltd. Evolution: Infectious speciation MICHAEL J. WADE Image Sources and Other Miscellaneous Sources • Slide 1: Sexist microbe. Wolbachia favor females, like this Ugandan butterfly, because they will carry on the lineage. CREDITS: COURTESY OF BACCHI AND BANDI; FRANCIS JIGGINS/CAMBRIDGE UNIVERSITY • • Wolbachia (2) http://www.xrefer.com/entry.jsp?xrefid=645055 (Symbiosis definition) Tree of Life: http://tolweb.org/tree?group=Eubacteria&contgroup=Life • Alpha-proteobacteria: http://www.life.umd.edu/classroom/bsci424/BSCI223WebSiteFiles/AlphaProteobacteria.htm • Filiaral worms: Zimmer, C. Wolbachia a tale of sex and survival. Science 292, 1093-1095 (2001). • Hosts in Wolbachia: Fruit fly www.mblab.gla.ac.uk/tubules/ gallery.html, Formica exsecta www.cs.unc.edu/~hedlund/ FormicaDBout.html, Ugandan butterfly, CREDITS: COURTESY OF BACCHI AND BANDI; FRANCIS JIGGINS/CAMBRIDGE UNIVERSITY, Filiaral worms: Zimmer, C. Wolbachia a tale of sex and survival. Science 292, 1093-1095 (2001), www.nhm.ac.uk/.../photos/ armadillidium_vulgare.html, Adalia bipunctata two-spot ladybug (ladybird) www.bio.umass.edu/biology/ alumni/biomass/vol03/ Wolbachia References • • • • • • Anderson, C.L., & Karr, T.L. Wolbachia: evolutionary novelty in a rickettisial bacteria. BMC Evolutionary Biology 1:10 (2001). URL: http://www.biomedcentral.com/1471-2148/1/10. Bordenstein, S.R., & Werren, J. Effects of A and B Wolbachia and host genotype on interspecies cytoplasmic incompatibility in Nasonia. Genetics 148, 1833-1844 (1998). Bordenstein, S.R., O’Hara, F.P., & Werren, J. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409, 707-710 (2001). Bourtzis, K., Dobson, S.L., Braig, H.R., & O’Neill, S.L. Rescuing Wolbachia have been overlooked… Nature 391, 852-853 (1998). Cordaux, R., Michel-Salzat, A., & Bouchon, D. Wolbachia infection in crustaceans; novel hosts and potential routes for horizontal infection. J. Evol. Biol. 14, 237-243 (2001). Dedeine, F., , Vavre F. *, Fleury,F., Loppin, B., Hochberg, M.E., & Boulétreau, M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA, 98, 6247-6252 (2001). Wolbachia References • • • • • • • Egas, M., Valia, F. & Breeuwer, J.A.J. On the evolution of cytoplasmic incompatibility in haplodiploid species. Evolution 56, 1101-1109 (2002). Huigens, M.E. et al. Infectious parthenogenesis. Nature 405, 178-179 (2000). Hurst, L.D., & Randerson, J.P. Parasitic sex puppeteers. Scientific American., 56-61 (April 2002). Jiggins, F.M., Hurst, G.D.D., & Majerus, M.E.N. Sex-ratio-distoring Wolbachia causes sex-role reversal in its butterfly host. Proc .R. Soc.Lond.B 267, 69-73 (2000). Jiggins, F.M., Hurst, G.D.D., Schuleburg, J.H. & Majerus, M.E.N. Two male-killing Wolbachia strains coexist within a population of butterfly Acraea encedon. Heredity 86, 161-166 (2001). Kageyama, D., Nishimura, G., Hoshizaki, S. & Ishikawa, Y. Feminizing Wolbachia in an insect, Ostrina furnacalis (Lepidoptera: Crambiae). Heredity 88, 444-449 (2002). Keller, L., et al. Sex-Ratio and Wolbachia in the ant Formica exsecta. Heredity 87, 227-233 (2001). Wolbachia References • • • • • • • • • • Ochman, H., & Moran, N.A. Genes lost and genes found: evolution of pathogenesis and symbiosis. Science 292, 1096-1098 (2001). Starr, D.J. & Cline, T.W. A host-parasite interaction rescues Drosophilia oogenesis defects. Nature 418, 76-79 (2002). Stouthamer, R., Breeuwer, J.A.J., & Hurst, G.D.D. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Ann. Rev. Microbiol. 53, 71-102 (1999). Taylor, M.J. & Hoerauf, A. Wolbachia bacteria of filiarial nemtodes. Parasitology Today 15,437-442 (1999). Tram, U. & Sullivan, W. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296, 1124-1126 (2002). Vavre, F. et al. Infection polymorphism and cytoplasmic incompatibility in Hymenoptera- Wolbachia associations. Heredity 88, 361-365 (2002). Wade, M.J. Infectious Speciation. Nature 409, 675-676 (2001). Werren, J.H. BIOLOGY OF WOLBACHIA. Annu. Rev. Entomol. 42:587-609 (1997). Zimmer, C. Wolbachia a tale of sex and survival. Science 292, 1093-1095 (2001). Zimmer, C. Timing is everything for Wolbachia hosts. Science 296, 999-1000 (2002).