Formulae of compounds
advertisement

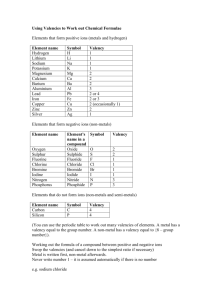
News! Match up the formulae Formulae of compounds Learning aims: Recognise common formulae Carry out flame tests 1 Formulas of elements and molecules • Individual elements are usually written as A compound when single atoms e.g. Na, is Mg, C, S, Fe more than one are written as • A few non-metallic elements element is chemically molecules containing two atoms: bonded to another • Hydrogen H2 element • Fluorine F2 etc. • It is important to be able to recognise common formulae 2 Some common compounds name formula atoms present HCl 1 H + 1 Cl sulphuric acid H2SO4 2H+1S+4O nitric acid HNO3 1H+1N+3O sodium hydroxide NaOH 1 Na + 1 O + 1 H KOH 1K+1O+1H carbon monoxide CO 1C+1O carbon dioxide CO2 1C+2O ammonia NH3 1 N + 3H nitrogen dioxide NO2 1N+2O sulphur dioxide SO2 1S+2O sulphur trioxide SO3 1S+3O hydrochloric acid potassium hydroxide 3 Have a read! The valency is the combining power of an element A positive valency ion or atom, will combine with a negatively charged one Take a few minutes to read pages 2 and 3 of your worksheet to look at common valencies of ions Think of the positive charged valencies as the first name, and the negatively charged as the surname, when naming a compound. 4 How do we go about writing the formula • • • • • • Write down the symbols from the table List the valency below each Cross them over Cancel them down if possible Write the formulae Let’s work through some examples… 5 Formula of compounds sodium oxide symbols Na O valency 1 2 formula Na2O 6 Formula of compounds lithium nitrate symbols Li NO3 valency 1 1 formula LiNO3 7 Formula of compounds copper(II) nitrate symbols Cu NO3 valency 2 1 formula Cu(NO3)2 8 Formula of compounds chromium(III) oxide symbols Cr O valency 3 2 formula Cr2O3 9 Formula of compounds ammonium sulphate symbols NH4 SO4 valency 1 2 formula (NH4)2SO4 10 Formula of compounds iron(II) hydroxide symbols Fe OH valency 2 1 formula Fe(OH)2 11 Formula of compounds 1 Nickel(II) iodate(V) Ni(IO3)2 Magnesium carbonate MgCO3 Potassium hydrogencarbonate KHCO3 Copper(II) sulphate CuSO4 Magnesium nitride Mg3N2 Zinc iodide ZnI2 Formula of compounds 2 Barium sulphite BaSO3 Lead(II) sulphate MgSO4 Sodium hydrogensulphate NaHSO4 Aluminium chloride AlCl3 Chromium(III) nitrate Cr(NO3)3 Iron(II) bromide FeBr2 13 Formula of compounds 3 Silicon chloride SiCl4 Ammonium phosphate (NH4)3PO4 Silver dichromate(VI) Ag2Cr2O7 Iron(III) oxide Fe2O3 Sodium nitrite NaNO2 Potassium manganate(VII) KMnO4 14 Break 15 Qualitative analysis Qualitative analysis is a method of analysis that tells us whether a substance is present or not. It does not tell us how much of something is there, so no numerical values Qualitative analysis of metal ions – flame tests Qualitative analysis of salts 16 Flame tests • When metals or salts containing those metals are heated in a flame characteristic colours are produced. This is used to identify certain metals. 17 Demo! 18 Flame tests Safety First: Wear safety goggles Stand up Tuck your chair & bags under your desk Tie back long hair 19 Formulae of compounds Learning aims: Recognise common formulae Carry out flame tests 20