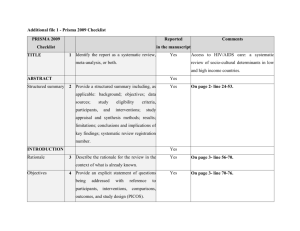
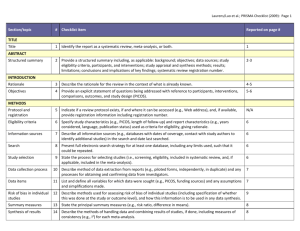
meta-analysis
advertisement

META-ANALYSIS AND SYSTEMATIC REVIEW Gianluigi Savarese, MD, FESC, ACC FIT Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden What is a Systematic Review? “A review that is conducted according to clearly stated, scientific research methods, and is designed to minimize biases and errors inherent to traditional, narrative reviews.” Kevin C. Chung, MD, Patricia B. Burns, MPH, H. Myra Kim, ScD, “Clinical Perspective: A Practical Guide to Meta-Analysis.” The Journal of Hand Surgery. Vol. 31A No.10 December 2006. p.1671 What is the significance of Systematic Reviews? • The large amount of medical literature requires clinicians and researchers alike to rely on systematic reviews in order to make an informed decision. • Systematic Reviews minimize bias. “A systematic review is a more scientific method of summarizing literature because specific protocols are used to determine which studies will be included in the review.” Margaliot, Zvi, Kevin C. Chung. “Systematic Reviews: A Primer for Plastic Surgery Research.” PRS Journal. 120/7 (2007) p.1839 Why are Systematic Reviews Necessary? “The volume of published material makes it impractical for an individual clinician to remain up to date on a variety of common conditions. This is further complicated when individual studies report conflicting conclusions, a problem that is prevalent when small patient samples and retrospective designs are used.” Margaliot, Zvi, Kevin C. Chung. “Systematic Reviews: A Primer for Plastic Surgery Research.” PRS Journal. 120/7 (2007) p.1839 Characteristics of Systematic Reviews • Two possible approaches: – or qualitative synthesis – statistical synthesis of data (meta-analysis) if appropriate and possible Hypothesis A systematic review should be based on principles of hypothesis testing, and the hypotheses must be conceived a priori. Four steps • Identify your studies • Determine eligibility of studies apriori to avoid bias – Inclusion: which ones to keep – Exclusion: which ones to throw out • Abstract Data from the studies • Analyze data in the studies statistically Literature Search A comprehensive and reproducible literature search is the foundation of a systematic review. Literature Search • Be methodical: plan first • List of popular databases to search – – – – – Pubmed/Medline Embase Cochrane Review ISI Web of Science SCOPUS Database bias!!! • Other strategies you may adopt – – – – – – – Trial registries (clinicaltrials.gov) Abstracts from meetings Hand search (go to the library...) Personal references References from published reviews/meta-analysis/trials Contact experts Web, eg. Google (http://scholar.google.com) Grey litterature Literature Search – Risk of Bias • English-language bias - occurs when reviewers exclude papers published in languages other than English • Citation bias - occurs when studies with significant or positive results are referenced in other publications, compared with studies with inconclusive or negative findings • Publication Bias - selective publication of articles that show positive treatment of effects and statistical significance. Data Collection • The list of data to be extracted should be decided a priori. • A data extraction form should be used so that the same data are extracted from each study and missing data are clearly apparent. • To be sure that data extraction is accurate and reproducible, it should be performed by at least two independent readers. • Disagreement between readers could be solved by agreements or by a third reviewer Margaliot, Zvi, Kevin C. Chung. “Systematic Reviews: A Primer for Plastic Surgery Research.” PRS Journal. 120/7 (2007) p.1839 Data Collection Collected data includes: – Study characteristics (year and journal of publication, number of patients in each arm, treatments performed, duration of follow-up) – Sample demographics (age, % males or females) – Sample characteristics (traditional CV risk factors - % hypertensive pts, % diabetic pts, % dyslipidemic pts, % smokers – concomitant treatments, comorbidities, etc) – Outcome data (all-cause death, CV death, MI, stroke, etc) Quality Assessment The validity of a systematic review ultimately depends on the scientific method of the retrieved studies and the reporting of data.” Margaliot, Zvi, Kevin C. Chung. “Systematic Reviews: A Primer for Plastic Surgery Research.” PRS Journal. 120/7 (2007) p.1839 GRADE Grading of Recommendations Assessment, Development and Evaluation Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ; GRADE Working Group. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008 May 3;336(7651):995-8. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ; GRADE Working Group. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008 May 3;336(7651):995-8. Data Synthesis Data could be summarized quantitatively if study designs are not too different in: • outcome definition (composite outcome?); • population sizes • population characteristics • interventions HETEROGENEITY Data Synthesis Once the data have been extracted and their quality and validity assessed, the outcomes of individual studies within a systematic review may be pooled and presented as summary outcome or effect META-ANALYSIS What is meta analysis? Quantitative approach for systematically combining results of previous research to arrive at conclusions about the body of research. What does it mean? • • • • • Quantitative : numbers Systematic : methodical combining: putting together previous research: what's already done conclusions: new knowledge Meta-analysis: Statistical Models • There are 2 statistical models used in a metaanalysis: – Fixed effects: 1. Effect of treatment is the same for every study; 2. Low heterogeneity – Random effects: 1. True effect estimate for each study varies; 2. High heterogeneity 3. Provide larger CI Heterogeneity • Clinical heterogeneity: variability in the participants, interventions and outcomes studied + • Methodological heterogeneity: variability in study design • Statistical heterogeneity: Variability in the intervention effects being evaluated in the different studies. It is a consequence of clinical or methodological diversity, or both, among the studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Heterogeneity assessment • If confidence intervals for the results of individual studies (generally depicted graphically using horizontal lines) have poor overlap, this generally indicates the presence of statistical heterogeneity. • Cochrane Q statistic: It is calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, with the weights being those used in the pooling method. A p value decided apriori defines the presence of significant heterogeneity. • I2 statistic: It describes the percentage of variation across studies that is due to heterogeneity rather than chance. 1. 0% to 40%: heterogeneity might not be important; 2. 30% to 60%: may represent moderate heterogeneity; 3. 50% to 90%: may represent substantial heterogeneity; 4. 75% to 100%: considerable heterogeneity. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Strategies for addressing heterogeneity • Check again that the data are correct • Do not do a meta-analysis • Explore heterogeneity (subgroup analysis, metaregression) • Ignore heterogeneity (there is no an intervention effect but a distribution of intervention effects) • Perform a random-effects meta-analysis (when heterogeneity cannot be explained) • Change the effect measure (different scales in different studies) • Exclude studies (outlying studies) Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Sensitivity analysis • One study removed meta-analysis • Meta-regression analysis Publication Bias Publication bias arises when trials with statistically significant results are more likely to be published and cited, and are preferentially published in English language journals and those indexed in Medline Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. International Journal of Epidemiology 2001;31:115-123. Publication Bias • A funnel plot is a simple scatter plot of the intervention effect estimates (OR, logOR) from individual studies against some measure of each study’s size or precision (standard error, 1/standard error, sample size, 1/sample size, log(sample size), log(1/sample size), Mantel-Haenszel weight). • The best choice of x axis for detecting the small sample effect is the log odds ratio. This is because the scale is not constrained and because the plot will be the same shape whether the outcome is defined as occurrence or nonoccurrence of event. Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54:1046-1055. Symmetrical plot in the absence of bias (open circles indicate smaller studies showing no beneficial effects) Asymmetrical plot in the presence of publication bias (smaller studies showing no beneficial effects are missing) Asymmetrical plot in the presence of bias due to low methodological quality of smaller studies (open circles indicate small studies of inadequate quality whose results are biased towards larger beneficial effects) Jonathan A et al. The Stata Journal 2004; 4:127 Publication Bias Ntot is the total sample size, NE and NC are the sizes of the experimental and control intervention groups, S is the total number of events across both groups and F = Ntot – S. Note that only the first three of these tests (Begg 1994, Egger 1997a, Tang 2000) can be used for continuous outcomes. Protocols The purpose of PRISMA (Preferred Reporting Items for Systematic Reviews and MetaAnalyses) guidelines is to provide proper procedures for conducting a meta-analysis and to standardize the methods of reporting a metaanalysis. Savarese G et al J Am Coll Cardiol 2013;61:131 Background • Angiotensin-converting enzyme inhibitors (ACE-Is) are recommended for reduction of cardiovascular (CV) events in patients at high CV risk without heart failure (HF). • In contrast, CV effects of angiotensin receptor blockers (ARBs) on major clinical outcomes in patients without HF are less certain as major clinical trials comparing ARBs vs placebo reported conflicting results. Savarese G et al J Am Coll Cardiol 2013;61:131 Methods – Inclusion Criteria • Report of at least one clinical outcome (all-cause death, CV death, myocardial infarction, stroke, new onset heart failure, new onset diabetes mellitus). • Randomized, placebo-controlled trials using ACE-Is or ARBs as treatments. Savarese G et al J Am Coll Cardiol 2013;61:131 Methods – Statistical methods • Meta-analysis was performed to assess the influence of treatments on outcomes. • Statistical homogeneity was assessed using Q statistic and further quantified with the I2 statistic. • Meta-regression was performed to test the influence of potential effect modifiers on results. • Publication bias was assessed using linear regression test by Egger and Macaskill’s modified test. Savarese G et al J Am Coll Cardiol 2013;61:131 Results – Search Strategy Savarese G et al J Am Coll Cardiol 2013;61:131 Results – Population characteristics Results – Population characteristics Results – Population characteristics Results – Composite Outcome ARBs significantly reduced the risk of the composite outcome by 7.0% compared to placebo (p=0.012). ACE-Is significantly reduced the risk of the composite outcome by 14.9% compared to placebo (p=0.001). Savarese G et al J Am Coll Cardiol 2013;61:131 Results – CV Death ARBs did not reduce the risk of CV death (p=0.768) 10% reduction of CV death did not achieve statistical significance in ACE-Is trials (p=0.087). Savarese G et al J Am Coll Cardiol 2013;61:131 Results – Myocardial infarction 9.5% reduction of MI risk did not achieve statistical significance in ARBs trials (p=0.086). ACE-Is significantly reduced the risk of MI by 17.7% (p<0.001) Savarese G et al J Am Coll Cardiol 2013;61:131 Results – Stroke ARBs significantly reduced the risk of stroke by 9.1% (p=0.011). ACE-Is significantly reduced the risk of stroke by 19.6% (p=0.004) Savarese G et al J Am Coll Cardiol 2013;61:131 Results – All Cause Death No significant effect was found on the risk of all-cause death in ARBs trials (p=0.866). ACE-Is reduced the risk of allcause death by 8.3% (p=0.008). Savarese G et al J Am Coll Cardiol 2013;61:131 Results – New onset HF No significant effect was found on the risk of new onset HF in ARBs trials (p=0.866). ACE-Is reduced the risk of new onset HF by 20.5% (p=0.001). Savarese G et al J Am Coll Cardiol 2013;61:131 Results – New onset DM ARBs significantly reduced the risk of new onset DM by 10.6% (p<0.01). ACE-Is reduced the risk of new onset diabetes by 13.7% (p=0.012) Savarese G et al J Am Coll Cardiol 2013;61:131 Results – Subgroup analysis Savarese G et al J Am Coll Cardiol 2013;61:131 Results – Meta-regression analysis Conclusions • In comparison to placebo, ACE-Is substantially reduce the composite of CV death/MI/stroke as well as allcause death, new onset HF and new onset DM in high-risk patients without HF, mostly with coronary or other vascular diseases. • ARBs, in high-risk patients mostly with DM or impaired glucose tolerance, without HF, reduce the composite outcome and new onset DM, but do not appear to reduce rates of all-cause death or new onset HF. Savarese G et al J Am Coll Cardiol 2013;61:131 Types of Meta-analysis/Terminology Systematic Review Meta-analysis (Overview) Extract data from published reports (aggregated data meta-analysis) Frequentist Approach Bayesian Approach Network Collect individual patient data (IPD) Frequentist approach Classical methods are, usually based on algorithms using explicit formulas. main assumptions of the model results of studies (usually RCTs) Transformations of input data Results of Meta-analysis Bayesian Meta-Analysis Including extra (prior) information main assumptions of the model establishing prior distributions basing on: Extra data e.g. results of non-randomized trials, historical observations, etc. Setting the level of conviction to this data ! MCMC simulations Results of Meta-analysis results of randomized studies Bayesian Meta-Analysis Assessing clinical significance main assumptions of the model non-informative prior distributions results of studies establishing the level of clinical significant result (e.g. RR > 1.2) MCMC simulations Results of Meta-analysis ! Answering the question: How probable is that the result is clinically significant? Possible to obtain due to knowledge of whole distribution Frequentist vs Bayesian approach Bayesian approach Frequentialist methods philosophy First: assumptions and construction then: inputing results of studies Construction based on the results of studies flexibility YES NO computation Makov Chain Monte Carlo simulations software specialistic, e.g. WinBUGS formulas no special requirements Network Meta-Analysis (Multiple Treatments Meta-Analysis, Mixed Treatment Comparisons) • Combine direct + indirect estimates of multiple treatment effects • Internally consistent set of estimates that respects randomization • Estimate effect of each intervention relative to every other whether or not there is direct comparison in studies • Calculate probability that each treatment is most effective • Compared to conventional pair-wise meta-analysis: • Greater precision in summary estimates • Ranking of treatments according to effectiveness 55 Indirect Comparisons of Multiple Treatments – Network Meta-Analysis Trial 1 A B 2 A B • Want to compare A vs. B Direct evidence from trials 1, 2 and 7 Indirect evidence from trials 3, 4, 5, 6 and 7 3 B C 4 B C 5 A C 6 A C 7 A B C • Combining all “A” arms and comparing with all “B” arms destroys randomization • Use indirect evidence of A vs. C and B vs. C comparisons as additional evidence to preserve randomization and within-study comparison Network Meta-Analysis (Multiple Treatments Meta-Analysis, Mixed Treatment Comparisons) paroxetine reboxetine duloxetine mirtazapine escitalopram fluvoxamine milnacipran citalopram sertraline venlafaxine bupropion fluoxetine milnacipran paroxetine sertraline bupropion fluvoxamine ? duloxetine escitalopram milnacipran 19 meta-analyses of pairwise comparisons published What is an individual patients data Meta-analysis? • Involves the central collection, checking and analysis of updated individual patient data • Include all properly randomised trials, published and unpublished • Include all patients in an intention-to-treat analysis Benefits of IPD • • • • • • • Carry out time-to-event analyses Only practical way to do subgroup analyses More flexible analysis of outcomes Carry out detailed data checking Ensure quality of randomisation and follow up Ensure appropriateness of analysis Update follow up information Other Benefits • • • • • • More complete identification of trials Better compliance in providing missing data More balanced interpretation of results Wider endorsement and dissemination of results Better clarification of further research Collaboration on further research THANKS FOR THE ATTENTION!!! Sorrento, Naples, Italy.