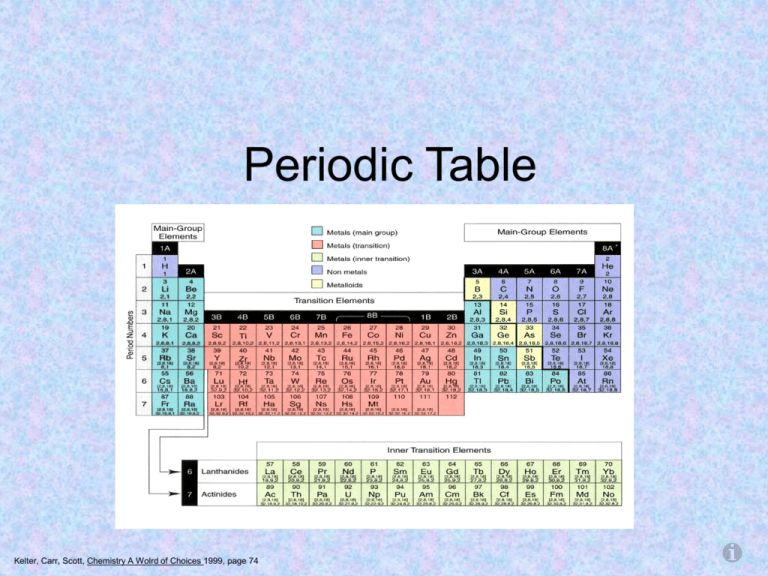
Periodic Table
Kelter, Carr, Scott, Chemistry A Wolrd of Choices 1999, page 74
Guiding Questions
Why is the periodic table so important?
Why is the periodic table shaped the way it's shaped?
Why do elements combine? Why do elements react?
What other patterns are there in the world and how do
they help us?
Table of Contents
‘Periodic Table’
How to Organize Elements
Mendeleev’s Periodic Table
Modern Periodic Table
Groups of Elements
Metals, Nonmetals, Metalloids
Discovering Elements
Origin of Names of Elements
Selected Elements
Electron Filling Order
Diatomic Molecules
Size of Atoms – Trends
Ionization Energy
Summary of Periodic Trends
Essential Elements
Element Project
Atomic Structure and Periodicity
You should be able to
Identify characteristics of and perform calculations with frequency and
wavelength.
Know the relationship between types of electromagnetic radiation and
Energy; for example, gamma rays are the most damaging.
Know what exhibits continuous and line spectra.
Know what each of the four quantum numbers n, l, m, and ms represents.
Identify the four quantum numbers for an electron in an atom.
Write complete and shorthand electron configurations as well as orbital
diagrams for an atom or ion of an element.
Identify the number and location of the valence electrons in an atom.
Apply the trends in atomic properties such as atomic radii, ionization
energy, electronegativity, electron affinity, and ionic size.
Calcium atom = [Ar]4s2
Potassium atom = [Ar]4s1
p = 20
n = 20
e = 20
p = 19
n = 20
e = 19
Ca 2 e- + Ca2+
K e- + K1+
Potassium ion = K1+ ≡ [Ar]
1s22s22p63s23p6
Calcium ion = Ca2+ ≡ [Ar] or
1s22s22p63s23p6
18e
19e19+
18e
20e-
>
20+
Oxygen atom = [He]2s22p4
Fluorine atom = [He] 2s22p5
p= 9
n = 10
e= 9
p= 8
n= 8
e= 8
F + e- F1-
O + 2 e- O2Oxide ion = O2- ≡ [Ne]
Oxygen
1s22s22p6
Fluoride
Fluorine ion = F1- ≡ [Ne]
1s22s22p6
8
6 e8+
8
7 e-
<
9+
Energy Level Diagram of a Many-Electron Atom
6s
6p
5d
4f
32
5s
5p
4d
18
4s
4p
3d
18
Arbitrary
Energy Scale
3s
3p
8
2s
2p
8
1s
2
NUCLEUS
O’Connor, Davis, MacNab, McClellan, CHEMISTRY Experiments and Principles 1982, page 177
How to Organize Elements…
Periodic Table Designs
How to Organize…
Baseball Cards:
year, team, player, card number, value ($).
Elements:
when they weremass,
discovered,
family, reactivity,
alphabetically,
value, density,
state or
of liquid
matter,ormetal
solid
gas vs. non-metal, atomic mass,
atomic number.
Which way is CORRECT to organize the elements?
Is it possible to organize the elements correctly in more than one way?
Interactive Periodic Table
e
Ir O N Mn
77
1
8
7
25
The Human Element
H
H
He
1
2
1
2
3
Li
Be
B
C
N
O
F
Ne
3
4
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
Na Mg
11
4
K
19
5
7
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
23
24
35
36
I
Xe
53
54
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
12
38
Cs Ba
55
56
Fr
Ra
87
88
*
W
25
43
26
44
Re Os
75
76
27
28
29
47
30
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
48
31
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Po At Rn
84
85
86
Rf Db Sg Bh Hs Mt
104
105
106
107
108
109
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Aliens Activity
Nautilus shell has a repeating pattern.
Look carefully at the drawings of the ‘aliens’.
Organize all the aliens into a meaningful pattern.
Aliens Lab
Cards
Periodic Table
Alkali earth metals
H
1
2
3
4
5
6
7
8A
He
Alkali metals
1A
Transition metals
3A 4A 5A 6A 7A
B C N O F
1
2A
Boron group
Li
Be
Nonmetals
3
4
Na
Mg
11
12
K
Ca
19
20
21
22
Rb
Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
37
38
39
40
41
42
49
Cs
Ba
Hf
Ta
W
55
56
72
73
74
Fr
Ra
87
88
Noble gases
5
Al
8B
3B 4B 5B 6B 7B
1B 2B
Sc Ti V Cr Mn Fe Co Ni Cu Zn
23
24
25
26
43
27
44
Re Os
75
76
28
29
30
47
13
45
46
48
Ir
Pt Au Hg
Tl
77
78
81
79
80
7
8
9
10
Si
P
S
Cl
Ar
14
15
16
17
18
As Se Br
Kr
33
32
Sn Sb
50
51
Pb Bi
82
83
34
35
36
Te
I
Xe
52
53
54
At
Rn
85
86
Po
84
Rf Db Sg Bh Hs Mt
104
105
106
107
108
109
Lanthanoid Series
6
C
Br Liquid
H
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
Solid
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Actinoid Series
7
Ac Th Pa
U
Np Pu Am Cm Bk Cf
Es Fm Md No Lr
Gas
89
90
91
92
93
94
95
96
97
98
99
Ne
6
Ga Ge
31
2
100
101
102
103
Dutch Periodic Table
117
116
115
114
113
112
111
110
109
108
107
106
Strong, Journal of Chemical Education, Sept. 1989, page 743
118
Stowe’s Periodic Table
Benfey’s Periodic Table
Döbereiner’s Triads
Johann Döbereiner
~1817
Name
Atomic
Mass
Name
Atomic
Mass
Name
Atomic
Mass
Calcium
Barium
40
137
Chlorine
Iodine
35.5
127
Sulfur
Tellurium
32
127.5
Average
88.5
Average
81.3
Average
79.8
Strontium
87.6
Bromine
79.9
Selenium
79.2
Döbereiner discovered groups of three related elements which he termed a triad.
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 161
Newlands Law of Octaves
John Newlands
~1863
Newlands Law of Octaves
1
2
3
4
5
6
7
Li
Na
K
Be
Mg
B
Al
C
Si
N
P
O
S
F
Cl
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 161
Development of Periodic Table
J.W. Döbereiner (1829)
Law of Triads
Elements could be classified into groups of three, or triads.
Trends in physical properties such as density, melting point,
and atomic mass were observed.
J.A.R. Newlands (1864)
Law of Octaves
Arranged the 62 known elements into groups of seven
according to increasing atomic mass.
He proposed that an eighth element would then repeat the
properties of the first element in the previous group.
Lothar Meyer (1830 – 1895)
Invented periodic table independently of Mendeleev
his work was not published until 1870 - one year after Mendeleev's
Dmitri Mendeleev
• Russian
• Invented periodic table
• Organized elements by
properties
• Arranged elements by atomic
mass
• Predicted existence of several
unknown elements
• Element 101
Dmitri Mendeleev
Dmitri Mendeléev
Mendeleev’s Periodic Table
Group I
II
III
IV
V
VI
VII
VIII
F = 19
Period
1
H=1
2
Li = 7
Be= 9.4
B = 11
C = 12
N = 14
O = 16
F = 19
3
Na = 23
Mg = 24
Al = 27.3
Si = 28
P = 31
S = 32
C = 35.5
4
K = 39
Ca = 40
? = 44
Ti = 48
V = 51
Cr = 52
Mn = 55
5
Cu = 63
Zn = 65
? = 68
? = 72
As = 75
Se = 78
Br = 80
6
Rb = 85
Sr = 87
? Yt = 88
Zr = 90
Nb = 94
Mo = 96
? = 100
7
Ag = 108
Cd = 112
In = 113
Sn = 118
Sb = 122
Te = 125
J = 127
8
Cs = 133
Ba = 137
?Di = 138
?Ce = 140
?Er = 178
?La = 180
Ta = 182
W = 184
Tl = 204
Pb = 207
Bi = 208
Fe =56, Co = 59,
Ni = 59
Ru= 104, Rh = 104,
Pd = 106
9
10
11
12
Au = 199
Hg = 200
Th = 231
U = 240
Os = 195, Ir = 197,
Pt = 198
Mendeleev’s Early Periodic Table
REIHEN
TABELLE II
GRUPPE I
___
Li = 7
K = 39
11
12
RH3
R2O5
Cs = 133
Sr = 87
GRUPPE VI
GRUPPE VII
RH2
RO3
In = 113
? Di = 138
__
__
(Au = 199)
__
? Yt = 88
Ba = 137
__
Si = 28
RH
R2O7
? Er = 178
Tl= 204
__
V = 51
Zr = 90
GRUPPE VIII
___
RO4
Cr = 52
Nb = 94
? Ce = 140
From Annalen der Chemie und Pharmacie, VIII, Supplementary Volume for 1872, p. 151.
__
W = 184
Pd = 106, Ag = 108
__ __ __ __
__
__
__
U = 240
Ni = 59, Cu = 63
Ru = 104, Rh = 104,
J = 127
__
Bi = 208
__
__ = 100
__
Ta = 182
Fe = 56, Co = 59,
Br = 80
Te = 125
__
Pb = 207
Mn = 55
Mo = 96
__
Cl = 35.5
Se = 78
Sb = 122
__
? La = 180
F = 19
S = 32
As = 75
Sn = 118
Th = 231
O = 16
P = 31
? = 72
__
__
Hg = 200
N = 14
Ti = 48
? = 68
__
Cd = 112
( __ )
__
Al = 27.3
Zn = 65
(Ag = 108)
C = 12
? = 44
__
Ca = 40
Rb = 85
9
10
GRUPPE V
RH4
RO2
B = 11
Mg = 24
(Cu = 63)
7
8
Be = 9.4
Na = 23
5
6
RO
R2O3
GRUPPE IV
H=1
3
4
GRUPPE III
___
R2O
1
2
GRUPPE II
___
Os = 195, Ir = 197,
__
__
Pt = 198, Au = 199
__ __ __ __
Elements Properties are Predicted
Property
Mendeleev’s Predictions in 1871
Observed Properties
Scandium (Discovered in 1877)
Molar Mass
Oxide formula
Density of oxide
Solubility of oxide
44 g
M2O3
3.5 g / ml
Dissolves in acids
43.7 g
Sc2O3
3.86 g / ml
Dissolves in acids
Gallium (Discovered in 1875)
Molar mass
Density of metal
Melting temperature
Oxide formula
Solubility of oxide
68 g
6.0 g / ml
Low
M2O3
Dissolves in ammonia solution
69.4 g
5.96 g / ml
30 0C
Ga2O3
Dissolves in ammonia
Germanium (Discovered in 1886)
Molar mass
Density of metal
Color of metal
Melting temperature
Oxide formula
Density of oxide
Chloride formula
Density of chloride
Boiling temperature
of chloride
72 g
5.5 g / ml
Dark gray
High
MO2
4.7 g / ml
MCl4
1.9 g / ml
Below 100 oC
O’Connor Davis, MacNab, McClellan, CHEMISTRY Experiments and Principles 1982, page 119,
71.9 g
5.47 g / ml
Grayish, white
900 0C
GeO2
4.70 g / ml
GeCl4
1.89 g / ml
86 0C
Periodic Table of the Elements
1
2
3
H
He
1
2
Li
Be
B
C
N
O
F
Ne
3
4
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
Na Mg
11
4
K
19
5
7
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
23
24
35
36
I
Xe
53
54
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
12
38
Cs Ba
55
56
Fr
Ra
87
88
*
W
25
43
26
44
Re Os
75
76
27
28
29
47
30
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
48
31
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Po At Rn
84
85
86
Rf Db Sg Bh Hs Mt
104
105
106
107
108
109
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Modern Periodic Table
• Henry G.J. Moseley
• Determined the atomic
numbers of elements
from their X-ray spectra
(1914)
• Arranged elements by
increasing atomic number
• Killed in WW I at age 28
(Battle of Gallipoli in Turkey)
1887 - 1915
Introduction to the Periodic Table
• Elements are arranged in seven horizontal rows, in order
of increasing atomic number from left to right and from
top to bottom.
• Rows are called periods and are numbered from 1 to 7.
• Elements with similar chemical properties form vertical
columns, called groups, which are numbered from 1 to
18.
• Groups 1, 2, and 13 through 18 are the main group
elements.
• Groups 3 through 12 are in the middle of the periodic
table and are the transition elements.
• The two rows of 14 elements at the bottom of the
periodic are the lanthanides and actinides.
Copyright 2007 Pearson Benjamin Cummings. All rights reserved.
Groups of Elements
1A
1
H
1
2
3
Be
3
4
7
2A
Alkali earth metals
6A
Oxygen group
Transition metals
7A
Halogens
3A
Boron group
8A
Noble gases
4A
Carbon group
8A
He
3A 4A
B C
5A 6A 7A 2
N O F Ne
Hydrogen
Inner transition metals
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
8B
K
3B 4B 5B 6B 7B
1B 2B 13 14 15 16 17
Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
12
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
Nitrogen group
Na Mg
19
5
5A
2A
Li
11
4
1A Alkali metals
38
Cs Ba
55
56
Fr
Ra
87
88
*
W
25
43
26
44
Re Os
75
76
27
28
29
47
30
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
48
31
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
35
36
I
Xe
53
54
Po At Rn
84
85
86
105
106
107
108
109
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
W
24
Kr
Rf Db Sg Bh Hs Mt
104
*
23
18
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Groups of Elements
1
18
He
2
13
14
15
16
17
2
Li
Be
N
O
F
Ne
3
4
7
8
9
10
Na
Mg
P
S
Cl
Ar
11
12
15
16
17
18
K
Ca
As
Se
Br
Kr
19
20
33
34
35
36
Rb
Sr
Sb
Te
I
Xe
37
38
51
52
53
54
Cs
Ba
Bi
Po
At
Rn
55
56
83
84
85
86
Fr
Ra
87
88
1
Alkali metals
16
Oxygen family
2
Alkaline earth metals
17
Halogens
18
Noble gases
15
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 367
Nitrogen family
Chemistry of the Groups
Group 16, the Chalcogens
– The chalcogens are oxygen, sulfur, selenium, tellurium, and
polonium.
16
O
8
S
All of the chalcogens have ns2np4 valence-electron configurations.
Their chemistry is dominated by three oxidation states:
1. –2, in which two electrons are added to achieve the
closed-shell electron of the next noble gas.
16
Se
34
Te
52
Po
2. +6, in which all six valence electrons are lost to give the
closed-shell electron configuration of the preceding noble
gas.
3. +4, in which only the four np electrons are lost to give a
filled ns2 subshell.
84
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Chemistry of the Groups
Group 15, the Pnicogens
– The pnicogens are nitrogen, phosphorus, arsenic, antimony, and
bismuth.
– All the pnicogens have ns2np3 valence-electron configurations,
leading to three common oxidation states:
15
N
7
P
15
As
1. –3, in which three electrons are added to give the
closed-shell electron configuration of the next noble gas
2. +5, in which all five valence electrons are lost to give the
closed-shell electron configuration of the preceding noble
gas
33
Sb
51
3. +3, in which only the three np electrons are lost to give a
filled ns2 subshell
Bi
83
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Chemistry of the Groups
Group 14
– Group 14 elements straddle the diagonal line that divides nonmetals from
metals.
– Carbon is a nonmetal, silicon and germanium are semimetals, and tin and
lead are metals.
– Group-14 elements have the ns2np2 valence-electron configuration.
– Group-14 elements have three oxidation states:
1. –4, in which four electrons are added to achieve the closed-shell
electron configuration of the next noble gas
2. +4, in which all four valence electrons are lost to give the closedshell electron configuration of the preceding noble gas
3. +2, in which the loss of two np2 electrons gives a filled ns2
subshell
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Chemistry of the Groups
Group 13
– Of the Group-13 elements, only the lightest, boron, lies on the
diagonal line that separates nonmetals and metals, it is a
semimetal and possesses an unusual structure.
– The rest of Group 13 are metals (aluminum, gallium, indium, and
thallium) and are typical metallic solids.
– Elements of Group 13 are highly reactive and form stable
compounds with oxygen.
– Elements of Group 13 have ns2np1 valence-electron configurations.
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Chemistry of the Groups
1A
1
2
3
H
1
2A
Li
Be
3
4
K
7
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
3B 4B 5B 6B 7B
1B 2B 13 14 15 16 17
Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
12
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
Transition Metals
5A 6A 7A 2
N O F Ne
8B
19
5
3A 4A
B C
Na Mg
11
4
8A
He
38
Cs Ba
55
56
Fr
Ra
87
88
Lanthanides
*
W
W
25
43
26
44
Re Os
75
76
27
28
29
47
30
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
48
31
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
35
36
I
Xe
53
54
Po At Rn
84
85
86
105
106
107
108
109
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
Actinides
24
Kr
Rf Db Sg Bh Hs Mt
104
*
23
18
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Metals and Nonmetals
1
2
3
H
He
1
2
Li
Be
B
C
3
4
5
Na Mg
11
4
K
19
5
7
Ca Sc
O
F
Ne
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
23
24
35
36
I
Xe
53
54
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
12
N
38
Cs Ba
55
56
Fr
Ra
87
88
*
W
Nonmetals
25
26
27
28
29
30
METALS
43
44
Re Os
75
76
47
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
48
31
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Po At Rn
84
85
86
Rf Db Sg Bh Hs Mt
104
105
106
107
108
Metalloids
109
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Metals, Nonmetals, & Metalloids
1
Nonmetals
2
3
4
5
Metals
6
7
Metalloids
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 349
Properties of Metals, Nonmetals,
and Metalloids
METALS
malleable, lustrous, ductile, good conductors of heat
and electricity
NONMETALS
gases or brittle solids at room temperature, poor
conductors of heat and electricity (insulators)
METALLOIDS (Semi-metals)
dull, brittle, semi-conductors (used in computer chips)
Discovering the Periodic Table
H
Li
Ancient Times
1894-1918
Midd. -1700
1923-1961
1735-1843
1965-
1843-1886
Be
B
C
N
O
F
Ne
Al
Si
P
S
Cl
Ar
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
Na Mg
K
Ca Sc
Rb Sr
Y
Cs Ba La
Fr
Ti
V
He
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
Hf
Tl
Pb Bi
Ta
W
Re Os
Ir
Pt Au Hg
I
Xe
Po At Rn
Ra Ac Rf Db Sg Bh Hs Mt
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa
Timeline of Elements Discovery
Journal of Chemical Education, Sept. 1989
U
Np Pu Am Cm Bk Cf Es Fm Md No Lr
Discovering the Periodic Table
H
Li
Ancient Times
1894-1918
Midd. -1700
1923-1961
1735-1843
1965-
1843-1886
Be
B
C
N
O
F
Ne
Al
Si
P
S
Cl
Ar
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
Na Mg
K
Ca Sc
Rb Sr
Y
Cs Ba La
Fr
Ti
V
He
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
Hf
Tl
Pb Bi
Ta
W
Re Os
Ir
Pt Au Hg
I
Xe
Po At Rn
Ra Ac Rf Db Sg Bh Hs Mt
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa
Journal of Chemical Education, Sept. 1989
U
Np Pu Am Cm Bk Cf Es Fm Md No Lr
Discovering the Periodic Table
H
Li
Ancient Times
1894-1918
Midd. -1700
1923-1961
1735-1843
1965-
1843-1886
Be
B
C
N
O
F
Ne
Al
Si
P
S
Cl
Ar
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
Na Mg
K
Ca Sc
Rb Sr
Y
Cs Ba La
Fr
Ti
V
He
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
Hf
Tl
Pb Bi
Ta
W
Re Os
Ir
Pt Au Hg
I
Xe
Po At Rn
Ra Ac Rf Db Sg Bh Hs Mt
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa
Journal of Chemical Education, Sept. 1989
U
Np Pu Am Cm Bk Cf Es Fm Md No Lr
Symbols are Useful
The use of symbols is not unique to chemistry.
Symbols can be quite helpful - when you know what they mean.
Arithmetic
+ - x ..
Money
$
Music
c
A Swedish chemist who invented modern chemical symbols.
Discovered the elements:
silicon, selenium, cerium, and thorium.
Jons Jakob Berzelius
(1799 - 1848)
Discovering the Elements
Metal
gold
silver
iron
mercury
tin
Sun
Moon
Mars
Mercury
Jupiter
Solie
Lunae
Martis
Mercurii
Jovis
lundi
mardi
mercredi
jeudi
Monday
Tuesday
Wednesday Thursday
copper lead
Symbol
Celestial body
Venus
Saturn
Day
Latin (dies)
French
dimanche
English
Sunday
Ringnes, Journal of Chemical Education, Sept. 1989, page 731
Veneris Saturni
vendredi
samedi
Friday
Saturday
Chemical Symbols
Gold
Sun
Silver
Moon
Iron
Mars
Copper
Venus
Lead
Saturn
Tin
Jupiter
Mercury
Mercury
Symbols
Ancient
used
Astronomical
in the 16th and
Symbols
17th Century
Fire
Air
Earth
Alchemical Symbols used in the 15th Century
Brownlee, Fuller, Hancock, Sohon, Whitsit, First Principles of Chemistry, 1931, page 74
Water
Chemical Symbols
Antimony
Water
Copper
Sulfur
Sulfuric acid
Symbols used in the 18th Century
Oxygen
Nitrogen
Copper
Hydrogen
Sulfur
Mercury
Carbon
Silver
Water
S
Carbon dioxide
Lead
C
Potassa
L
Alcohol
Symbols used by John Dalton
Brownlee, Fuller, Hancock, Sohon, Whitsit, First Principles of Chemistry, 1931, page 74
Soda
Gold
G
Origin of the Names of Elements
Title
Pre-chemical Names
Names from celestial bodies
Names from mythology / superstition
Names from minerals / ores,
other than geographical names
Names from colors
Names from properties other than color
Geographical names from the domicile or
workplace of the discoverer(s)
Geographical names from minerals / ores
Constructed names
Names from persons
Ringnes, Journal of Chemical Education, Sept. 1989, page 731
Number of Elements
10
8
10
13
9
8
13
10
16
10
Map of Elements Discovered
Ringnes, Journal of Chemical Education, Sept. 1989, page 732
Several Synthetic Elements
Synthetic
•
•
•
•
Man-made
Bk = Berkelium
Cf = Californium
Am = Americium
–
All made by nuclear bombardment
at Berkeley, California, U.S.A.
Einsteinium (Es)
Albert Einstein
– Relativity
– E = mc2
– Offered Presidency of Israel
– Element 99
– Photoelectric effect
• Solar calculator
Curium (Cm)
• Madame Curie
– Pioneer in radioactivity
• (Ra = radium)
– 25 pounds of pitchblende ore
yields 1/1000 of a gram of
radium
– Emits 2 millions times as much
radiation as uranium
• (Rn = radon gas)
– Discovered 5 elements
– Nobel Prize (5 in Curie family)
– Born in Poland
• (Po = polonium)
Marie Curie (1876–1934)
Radium (Ra)
Radium was used as a fluorescent paint on watch dials. It was
applied with thin brushes that workers would lick to keep a fine tip.
Many people died from the exposure to radium.
Radon Gas
Zone 1 counties have a predicted average indoor radon screening
level greater than 4 pCi/L (pico curies per liter) (red zones)
Zone 2 counties have a predicted average indoor radon screening
level between 2 and 4 pCi/L (orange zones)
Zone 3 counties have a predicted average indoor radon screening
level less than 2 pCi/L (yellow zones)
Radon gas occurs naturally
from the radioactive decay
of radium. Radium is found
in small amounts in rock.
Ra Rn + radiation
Predicted fraction of homes over 4 picocuries/liter radon
http://www.epa.gov/radon/zonemap.html
Nobelium (No)
Element 102
Inventor:
dynamite (TNT)
blasting gelatin
Nobel Prize
NO2
O2N
NO2
CH3
Trinitrotoluene
Alfred Nobel
“Merchant of Death”
Seaborgium (Sg)
Glenn Seaborg
– Separated f-block from rest of periodic table
– Worked on Manhattan Project
(Atomic bomb)
– Classified until after WW II
– Element 106
• Only living person to have an element named for
them
Silicon vs. Silicone
• Silicon (Si) element
• Silicone (…Si – O – Si…) polymer
– Sealant (caulk) prevents leaks
– Breast augmentation
No cause-and-effect relationship exists between
breast enlargement and breast cancer. Only one
researcher found a causal link.
12
Mg
Magnesium
Atomic Mass 24 amu
melting point =
silver gray metal
used in flash bulbs, bombs,and flares
8th most abundant element (2.2% of Earth’s crust)
lack of Mg produces same biological effect
as alcoholism (delirium tremens)
24.305
Potassium Metal in Water
Newmark, CHEMISTRY, 1993, page 25
The Periodic Table
1
Alkaline
earth metals
Noble
gases
Halogens
18
H
He
2
13
14
15
16
17
Li
Be
B
C
N
O
F
Ne
3
4
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
1
3
Na Mg
Alkali metals
11
K
19
4
5
6
7
8
9
Transition metals
10
11
12
12
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
23
24
35
36
I
Xe
53
54
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
*
Hf
Ta
W
72
73
74
Y
Rf Db Sg Bh Hs Mt Uun Uuu Uub
37
38
Cs Ba
55
56
Fr
Ra
87
88
* Lanthanides
104
La
57
Y Actinides
2
Ac
89
105
106
25
43
26
44
Re Os
75
107
76
108
27
28
29
46
Ir
Pt Au Hg
Tl
77
78
81
110
79
111
48
31
45
109
47
30
80
112
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Po At Rn
84
85
86
Uuq
Uuh
Uuo
113
116
118
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
58
59
60
Th Pa
U
90
92
91
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Orbitals Being Filled
1
Periods
1
1s
8
Groups
2
3
4
5
2
2s
2p
3
3s
3p
4
4s
3d
4p
5
5s
4d
5p
6
6s
La
5d
6p
7
7s
Ac
6d
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 345
6
7 1s
4f
Lanthanide series
5f
Actinide series
Electron Filling in Periodic Table
s
p
1
2
d
3
4
5
6
*
7
W
f
*
W
s
Electron Filling in Periodic Table
metallic character increases
nonmetallic character increases
metallic character increases
nonmetallic character increases
Periodic Table
s
1
s
H
p
H
He
1
2
1
2
3
Li
Be
B
C
N
O
F
Ne
3
4
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
Na Mg
11
4
K
19
5
7
12
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
23
24
35
36
I
Xe
53
54
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
d
38
Cs Ba
55
56
Fr
Ra
87
88
*
W
25
43
26
44
Re Os
75
76
27
28
29
30
47
48
31
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Po At Rn
84
85
86
Rf Db Sg Bh Hs Mt
104
105
106
107
108
109
f
*
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
W
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Melting Points
He
1
H
Mg
-259.2
2
3
4
Li
Be
180.5
1283
650
K
Ca Sc
Rb Sr
38.8
6
> 3000
98
850
770
710
B
oC
2000 - 3000
oC
Al
660
Ti
V
C
N
O
F
Y
1500 1852 2487 2610 2127 2427 1966 1550
920
Ta
P
1423 44.2
420 29.78 960
Zr Nb Mo Tc Ru Rh Pd Ag Cd
Hf
Si
S
119
Ne
W
Re Os
Ir
961
In
Ar
-101 -189.6
Kr
817 217.4 -7.2 -157.2
Sn Sb Te
I
Xe
321 156.2 231.9 630.5 450 113.6 -111.9
Pt Au Hg
Tl
Pb Bi
Po At Rn
2222 2997 3380 3180 2727 2454 1769 1063 -38.9 303.6 327.4 271.3 254
Ralph A. Burns, Fundamentals of Chemistry , 1999, page 1999
Cl
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
1423 1677 1917 1900 1244 1539 1495 1455 1083
Cs Ba La
28.6
-269.7
2027 4100 -210.1 -218.8 -219.6 -248.6
Na Mg
63.2
5
650
He
0.126
Symbol
Melting point oC
-71
Elements with Highest Densities
Element
Osmium
Iridium
Platinum
Rhenium
Neptunium
Plutonium
Gold
Tungsten
Uranium
Tantalum
Year
Discovered
1804
1804
1784
1925
1940
1940
prehistoric
1783
1789
1802
Density
(g/cm3)
22.59
22.56
21.45
21.01
20.47
20.26
19.32
19.26
19.05
16.67
Densities of
Elements
1
2
3
4
H
He
0.071
0.126
Li
Be
B
C
N
O
0.53
1.8
2.5
2.26
0.81
1.14
Na Mg
Al
Si
P
S
0.97
2.70
2.4 1.82w 2.07 1.557 1.402
K
0.86
5
Ca Sc
Ti
V
1.55
4.5
5.96
Rb Sr
(2.5)
2.6
I
Xe
4.93
3.06
7.86
8.9
8.90
8.92
7.14
5.91
5.36
5,7
4.7
6.4
8.4
10.2
8.6
7.3
7.3
Cs Ba La
Hf
Ta
W
Pt Au Hg
Tl
Pb Bi
1.90
13.1
16.6
19.3
8.0 – 11.9 g/cm3
Mg
1.74
W
Ar
3.119
7.4
5.51
6.7
Cl
7.1
Sn Sb Te
3.5
1.11 1.204
Kr
In
2.6
Y
Ne
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Zr Nb Mo Tc Ru Rh Pd Ag Cd
1.53
6
1.74
F
11.5
12.5
Re Os
12.5
Ir
12.0
10.5
21.4 22.48 22.4 21.45 19.3 13.55 11.85 11.34
12.0 – 17.9 g/cm3
6.7
9.8
6.1
Po At Rn
9.4
> 18.0 g/cm3
Symbol
Density in g/cm3C, for gases, in g/L
---
4.4
4f
Sublevels
4d
Energy
n=4
n=3
4p
3d
4s
3p
3s
2p
n=2
2s
n=1
1s
1
H
H
He
1
2
1
2
3
Li
Be
B
C
N
O
F
Ne
3
4
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
Na Mg
11
4
K
19
5
7
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
23
24
35
36
I
Xe
53
54
20
21
22
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
39
40
41
42
49
Hf
Ta
W
72
73
74
37
6
12
38
Cs Ba
55
56
Fr
Ra
87
88
*
W
25
43
26
44
Re Os
75
76
27
28
29
47
30
45
46
Ir
Pt Au Hg
Tl
77
78
81
79
48
31
80
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Po At Rn
84
85
86
Rf Db Sg Bh Hs Mt
104
105
106
107
108
109
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
57
58
59
Ac Th Pa
89
90
91
60
U
92
61
62
63
64
65
66
Np Pu Am Cm Bk Cf
93
94
95
96
97
98
67
68
69
70
71
Es Fm Md No Lr
99
100
101
102
103
Electron Filling in Periodic Table
s
s
1
H
p
H
He
1s1
1s2
1s1
2
3
4
5
6
7
Li
Be
B
C
N
O
F
Ne
2s1
2s2
2p1
2p2
2p3
2p4
2p5
2p6
Al
Si
P
S
Cl
Ar
3p1
3p2
3p3
3p4
3p5
3p6
Na Mg
d
3s1
3s2
K
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
Kr
4s1
4s2
3d1
3d2
3d3
3d5
3d10
4p1
4p2
4p5
4p6
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
I
Xe
5s1
4d1
4d2
4d4
4d5
4d6
4d7
4d8
4d10
4p1
5p1
5p2
5p5
5p6
Hf
Ta
W
Re Os
Ir
Pt Au Hg
Tl
Pb Bi
Po At Rn
5d2
5d3
5d4
5d5
5d7
5d9
6p1
6p2
6p4
5s2
Cs Ba
6s1
6s2
Fr
Ra
7s1
7s2
*
W
3d5
3d6
5d6
3d7
3d8
3d10
4d10
5d10
5d10
4p3
5p3
6p3
4p4
5p4
6p5
6p6
Rf Db Sg Bh Hs Mt
6d2
6d3
6d4
6d5
6d6
6d7
f
*
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
5d1
W
4f2
4f3
4f4
Ac Th Pa
U
6d1
5f3
6d2
5f2
4f5
4f6
4f7
4f7
4f9
4f10
Np Pu Am Cm Bk Cf
5f4
5f6
5f7
5f7
5f8
5f10
4f11
4f12
4f13
4f14
4f114
Es Fm Md No Lr
5f11
5f14
5f13
5f14
5f14
Names and Symbols of
Selected Elements
Name*
Symbol
Name*
Symbol
Aluminum
Argon
Barium
Boron
Bromine
Cadmium
Calcium
Carbon
Chlorine
Cobalt
Copper (cuprum)
Fluorine
Gold (aurum)
Helium
Hydrogen
Iodine
Iron (ferrum)
Al
Ar
Ba
B
Br
Cd
Ca
C
Cl
Co
Cu
F
Au
He
H
I
Fe
Lead (plumbum)
Lithium
Magnesium
Mercury (hydrargyrum)
Neon
Nickel
Nitrogen
Oxygen
Phosphorus
Potassium (kalium)
Silicon
Silver (argentum)
Sodium (natrum)
Strontium
Sulfur
Tin (stannum)
Zinc
Pb
Li
Mg
Hg
Ne
Ni
N
O
P
K
Si
Ag
Na
Sr
S
Sn
Zn
*Names given in parentheses are ancient Latin or Greek words from which the symbols are derived.
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Electronegativity
The ability of an
atom in a molecule
to attract shared
electrons to itself.
Linus Pauling
1901 - 1994
Electronegativities
1A
1
Period
2
3
4
5
6
7
8A
H
2.1
2A
3A
4A
5A
6A
7A
Li
Be
B
C
N
O
F
1.0
1.5
2.0
2.5
3.0
3.5
4.0
Al
Si
P
S
Cl
1.5
1.8
2.1
2.5
3.0
Na Mg
1.2
3B
4B
5B
6B
K
Ca Sc
Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
0.8
1.0
1.3
1.5
1.6
1.6
1.7
1.6
1.8
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
0.8
1.2
1.4
1.6
1.8
1.9
2.2
2.2
2.2
1.7
1.7
1.8
Cs Ba
La*
Hf
Ta
W
Re Os
Ir
Pt Au Hg
Tl
Pb Bi
Po At
0.7
1.1
1.3
1.5
1.7
1.9
2.2
2.2
1.8
1.8
2.0
1.0
0.9
y
Fr
Ra Ac
0.7
0.9
1.1
8B
7B
1.5
1.8
2.2
1.8
1B
2B
0.9
1.8
1.9
1.9
2.4
1.9
2.0
1.9
1.9
2.4
2.1
* Lanthanides: 1.1 - 1.3
yActinides:
1.3 - 1.5
Hill, Petrucci, General Chemistry An Integrated Approach 2nd Edition, page 373
Below 1.0
2.0 - 2.4
1.0 - 1.4
2.5 - 2.9
1.5 - 1.9
3.0 - 4.0
2.8
I
2.5
2.2
Covalent Bonds
Polar-Covalent bonds
Electrons are unequally shared
Electronegativity difference between 0.3 and 1.7
Example: H2O (water)
O = 3.5
H = 2.1
difference is 1.4
Nonpolar-Covalent bonds
Electrons are equally shared
Electronegativity difference of 0 to 0.3
Nitrogen gas molecules
A Collection of Argon Atoms
Oxygen gas molecules
Diatomic Molecules
Hydrogen (H2)
atomic radius = 37 pm
Distance
between
nuclei
Nucleus
Chlorine (Cl2)
atomic radius = 99 pm
Fluorine (F2)
atomic radius = 64 pm
Bromine (Br2)
atomic radius = 114 pm
Atomic
radius
Oxygen (O2)
atomic radius = 66 pm
Nitrogen (N2)
atomic radius = 71 pm
HOBrFINCl twins
Iodine (I2)
atomic radius = 138 pm
H2 O2 Br2 F2 I2 N2 Cl2
Diatomic Molecules
Elements That Exist as Diatomic Molecules in Their Elemental Forms
Element Present
hydrogen
nitrogen
oxygen
fluorine
chlorine
bromine
iodine
Elemental State at 25 oC
colorless gas
colorless gas
pale blue gas
pale yellow gas
pale green gas
reddish-brown liquid
lustrous, dark purple solid
Molecule
H2
N2
O2
F2
Cl2
Br2
I2
Atomic Radii
IA
IIA
IIIA
IVA
VA
VIA
VIIA
Li
Be
B
C
N
O
F
1.52
1.11
0.88
0.77
0.70
0.66
0.64
Na
Mg
Al
Si
P
S
Cl
1.86
1.60
1.43
1.17
1.10
1.04
0.99
K
Ca
Ga
Ge
As
Se
Br
2.31
1.97
1.22
1.22
1.21
1.17
1.14
Rb
Sr
In
Sn
Sb
Te
I
2.44
2.15
1.62
1.40
1.41
1.37
1.33
Cs
Ba
Tl
Pb
Bi
2.62
2.17
1.71
1.75
1.46
= 1 Angstrom
0.3
Cs
Rb
atomic radius
0.25
K
0.2
Na
4d
transition
series
3d
transition
series
Li
0.15
La
Zn
Xe
Kr
0.1
Cl
F
0.05
He
H
0
0
10
20
30
atomic number
40
50
60
Periodic Trends in Atomic Radii
LeMay Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 175
Relative Size of Atoms
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 350
Attraction and Repulsion of
Electrical Charges
+
-
Particles with opposite
charges attract
one another.
+ +
-
Particles with like charges
repel one another.
-
Coulombic Attraction
A
1+
1D
B
C
2+
2+
2-
4-
3-
Coulombic Attraction
2-
1) Charge
opposites attract
like repels
2) Distance
Shielding Effect
Valence
+
nucleus
-
Kernel electrons block
the attractive force of
the nucleus from the
valence electrons
-
Electron
Shield
“kernel”
electrons
Electrons
Shielding Effect and
Effective Nuclear Charge
12
Mg
24.305
attractions
repulsions
+
_
_
_
Mg = [Ne]3s2
Hill, Petrucci, General Chemistry An Integrated Approach 2nd Edition, page 336
Decreasing Atomic Size
Across a Period
• As the attraction between the (+) nucleus and the (–) valence electrons ,
the atomic size . Greater coulombic attraction.
• From left to right, size decreases because there is an increase in nuclear
charge and Effective Nuclear Charge (# protons – # core electrons).
• Each valence electron is pulled by the full ENC
Li
Be
B
1s22s1
1s22s2
1s22s22p1
(ENC = 1)
(ENC = 2)
(ENC = 3)
Li
Be
B
++
+
++
+
+
+++
++
Sizes of ions: electron repulsion
• Valence electrons repel each other.
• When an atom becomes a
anion (adds an electron to its
valence shell) the repulsion
between valence electrons
increases without changing ENC
• Thus, F– is larger than F
- 9+
- Fluorine atom
F
2
1s 2s22p5
+1e-
-
9+
9+
-
-
Fluorine ion
Fluoride
F11s22s22p6
Atomic Radius of Atoms
Be
B
C
Na Mg
Al
Si
K
Ca
Ga
Ge
Rb
Sr
In
Sn
Sb
Tl
Pb
Bi
Cs
Ba
O
F
P
S
Cl
As
Se
Br
N
Te
I
Atomic
Ionic Radii
Radii
IA
IIA
IIIA
IVA
Li1+
Li
VA
VIA
Be2+
Be
B
C
NN3-
OO2-
F1F
1.52
0.60
1.11
0.31
0.88
0.77
0.70
1.71
0.66
1.40
0.64
1.36
1+
Na
Na
Mg2+
Mg
Al3+
Al
Si
P
1.43
0.50
1.17
1.10
1.04
1.84
0.99
1.81
2SS
VIIA
1ClCl
1.86
0.95
1.60
0.65
K
K1+
Ca
Ca2+
Ga3+
Ge
As
Se2Se
Br1Br
2.31
1.33
1.97
0.99
1.22
0.62
1.22
1.21
1.17
1.98
1.14
1.85
Rb
Rb1+
Sr
Sr2+
In3+
In
Sn
Sb
2.44
1.48
2.15
1.13
1.62
0.81
1.40
1.41
Tl3+
Tl
Pb
Bi
1.71
0.95
1.75
1.46
Cs
Cs1+
Ba
Ba2+
2.62
1.69
2.17
1.35
2TeTe
1.37
2.21
II1-
1.33
2.16
= 1 Angstrom
= 1 Angstrom
Atomic Radii
VIIIA
VIIA
He
IA
IIA
IIIA
IVA
VA
VIA
Li
Be
B
C
N
O
F
Ne
1.52
1.11
0.88
0.77
0.70
0.66
0.64
1.12
Na
Mg
Al
Si
P
S
Cl
Ar
1.86
1.60
1.43
1.17
1.10
1.04
0.99
1.54
K
Ca
Ga
Ge
As
Se
Br
Kr
2.31
1.97
1.22
1.22
1.21
1.17
1.14
1.69
Rb
Sr
In
Sn
Sb
Te
I
Xe
2.44
2.15
1.62
1.40
1.41
1.37
1.33
1.90
Cs
Ba
Tl
Pb
Bi
Rn
2.62
2.17
1.71
1.75
1.46
2.20
0.93
Ionic Radii
IA
IIA
Li1+
Be2+
0.60
0.31
Na1+
Mg2+
0.95
0.65
K1+
Ca2+
1.33
0.99
IIIA
Sr2+
1.48
1.13
VA
VIA
VIIA
N3-
O2-
F1-
1.71
1.40
1.36
S2-
Cl1-
1.84
1.81
Ga3+
Se2-
Br1-
0.62
1.98
1.85
Al3+
0.50
In3+
Rb1+
IVA
0.81
Te22.21
I12.16
Tl3+
Cs1+
Ba2+
1.69
1.35
0.95
= 1 Angstrom
Trends in Atomic and Ionic Size
Metals
Nonmetals
Group 1
Group 13
Group 17
e
e
Li+
Li
152
F
64
60
e
e
Na+
Na
95
e
e
136
e
Al3+
Al
143
F-
50
Cl
Cl-
99
186
181
e
e
K+
Br
K
227
133
Cations are smaller than parent atoms
114
Br195
Anions are larger than parent atoms
e
Li+
Li
152
60
e
e
Li+
e
Li
Li +
e
Lithium ion
152
Lithium atom
152
Lithium atom
60
IA
Atomic
Radii
Li
1.52
Na
1.86
K
2.31
Rb
2.44
Cs
Ionic
Radii
IIIA
IVA
Be
B
C
0.88
0.77
Al
Si
1.60
1.43
1.17
Ca
1.97
Ga
Ge
1.22
Sr
1.11
Mg
VA
VIA
VIIA
N
O
F
0.70
P
0.66
S
0.64
Cl
1.10
1.04
0.99
As
Se
Br
1.22
1.21
1.17
1.14
In
Sn
Sb
Te
2.15
1.62
1.40
1.41
I
1.33
Ba
Tl
Pb
Bi
1.71
1.75
1.46
2.62
2.17
Li1+
Be2+
0.60
Na1+
0.31
0.95
0.65
K1+
Cations: smaller
than parent atoms
IIA
Mg2+
Ca2+
N31.71
Al3+
0.50
Ga3+
1.33
Rb1+
0.99
Sr2+
0.62
1.48
Cs1+
1.13
Ba2+
0.81
Tl3+
1.69
1.35
0.95
In3+
1.37
O21.40
F11.36
S21.84
Cl11.81
Se2-
Br1-
1.98
1.85
Te2-
I1-
2.21
2.16
= 1 Angstrom
Anions: LARGER
than parent atoms
The Octet Rule and Common Ions
-
-
8+
-
-
-
-
Oxygen atom
O
2
1s 2s22p4
+2e-
-
-
-
- 9+
- -
- - 10+
- -
- - 11+
- -
Fluorine atom
F
2
1s 2s22p5
Neon atom
Ne
2
1s 2s22p6
Sodium atom
Na
2
1s 2s22p63s1
+1e-
-1e-
-
- - 12+
- Magnesium atom
Mg
2
1s 2s22p63s2
-2e-
8+
-
- 9+
- -
- - 11+
- -
- - 12+
- -
Oxygen ion
O21s22s22p6
Fluorine ion
F11s22s22p6
Sodium ion
Na1+
1s22s22p6
Magnesium ion
Mg2+
1s22s22p6
-
Isoelectronic Species
Isoelectronic - all species have the same number of electrons.
p=8
n=8
e = 10
p=9
n=9
e = 10
p = 10
n = 10
e = 10
p = 11
n = 11
e = 10
p = 12
n = 12
e = 10
8+
-
- 9+
- -
- - 10+
- -
- - 11+
- -
- - 12+
- -
Oxygen ion
O21s22s22p6
Fluorine ion
F11s22s22p6
Neon atom
Ne
2
1s 2s22p6
Sodium ion
Na1+
1s22s22p6
Magnesium ion
Mg2+
1s22s22p6
-
-
Can you come up with another isoelectronic series of five elements?
Lewis Structure
“Lewis Dot Notation”
o o
Na Cl
o
X
o
o
o o
Na Cl
Na Cl
X D
X D
X D
HC N
o
X
D
D
H C N
H C N
Gilbert Lewis
Atomic Radius vs. Atomic Number
0.3
Cs
Rb
atomic radius
0.25
K
0.2
Na
4d
transition
series
3d
transition
series
Li
0.15
La
Zn
Xe
Kr
0.1
Cl
F
0.05
He
H
0
0
10
20
30
atomic number
40
50
60
Hungry for Tater Tots?
Mr. C at 7 years old.
OUCH!!
Ionization Energies
18
Group 1
1
Period
2
3
H
6
7
13
14
15
16
17
B
C
N
O
F
2
Li
Be
520
900
801
Na Mg
Al
Si
578
787
738
12
S
Cl
Ar
590
633
659
651
906
579
762
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd
In
Sn Sb Te
403
600
640
652
684
702
868
558
709
Cs Ba
La*
Hf
Ta
W
Re Os
Pt Au Hg
Tl
Pb Bi
Po At Rn
376
538
659
761
770
760
868
589
716
812
Fr
--
503
11
P
V
550
10
1086 1402 1314 1681 2081
Ti
7
9
Ne
Ca Sc
K
3
8
2372
5
738
6
First Ionization Energy
(kJ/mol)
4
419
5
He
Symbol
1312
496
4
Mg
1012 1000 1251 1521
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
653
717
762
710
839
760
720
Ir
878
737
804
746
731
890
1007
947
834
703
941
869
Kr
1140 1351
I
Xe
1008 1170
--
1038
y
Ra Ac Rf Db Sg Bh Hs Mt Ds Uuu Uub Uut Uuq Uup
509
490
--
* Lanthanide series
--
series
--
--
--
--
--
--
--
--
--
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
534
y Actinide
--
527
Th Pa
587
570
533
U
598
536
545
547
592
566
573
581
589
597
603
523
Np Pu Am Cm Bk Cf
Es Fm Md No Lr
600
619
585
578
581
601
608
627
635
642
--
First Ionization Energies
(in kilojoules per mole)
H
He
1312.1
2372.5
Li
Be
B
C
N
O
F
Ne
520.3
899.5
800.7
1086.5
1402.4
1314.0
1681.1
2080.8
Na
Mg
Al
Si
P
S
Cl
Ar
495.9
737.8
577.6
786.5
1011.8
999.7
1251.2
1520.6
K
Ca
Ga
Ge
As
Se
Br
Kr
418.9
589.9
578.6
761.2
946.5
940.7
1142.7
1350.8
Rb
Sr
In
Sn
Sb
Te
I
Xe
402.9
549.2
558.2
708.4
833.8
869.0
1008.7
1170.3
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 188
First Ionization Energies
(kJ/mol)
s
p
H
He
1312.1
2372.5
Li
Be
B
C
N
O
F
Ne
520.3
899.5
800.7
1086.5
1402.4
1314.0
1681.1
2080.8
Na
Mg
Al
Si
P
S
Cl
Ar
495.9
737.8
577.6
786.5
1011.8
999.7
1251.2
1520.6
K
Ca
Ga
Ge
As
Se
Br
Kr
418.9
589.9
578.6
761.2
946.5
940.7
1142.7
1350.8
Rb
Sr
In
Sn
Sb
Te
I
Xe
402.9
549.2
558.2
708.4
833.8
869.0
1008.7
1170.3
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 188
First Ionization Energies
Metal
Metalloid
Nonmetal
(kJ/mol)
s
p
H
He
1312.1
2372.5
Li
Be
B
C
N
O
F
Ne
520.3
899.5
800.7
1086.5
1402.4
1314.0
1681.1
2080.8
Na
Mg
Al
Si
P
S
Cl
Ar
495.9
737.8
577.6
786.5
1011.8
999.7
1251.2
1520.6
K
Ca
Ga
Ge
As
Se
Br
Kr
418.9
589.9
578.6
761.2
946.5
940.7
1142.7
1350.8
Rb
Sr
In
Sn
Sb
Te
I
Xe
402.9
549.2
558.2
708.4
833.8
869.0
1008.7
1170.3
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 188
First Ionization energy
He
• Helium (He) has…
n
• a greater IE than H
• same shielding
• greater nuclear charge
H
1e-
2e-
1+
2+
H
He
Atomic number
First Ionization energy
He
n
•
Li has…
lower IE than H
more shielding
•
Further away outweighs
•
•
H
greater nuclear charge
Li
Atomic number
First Ionization energy
He
n
H
Be has higher IE than Li
same shielding
greater nuclear charge
2e-
Be
2e1e- -
3+
3+ 2e 1e
Li
Li
Atomic number
2e4+4+2e- 2e-
Be
First Ionization energy
He
n
B has lower IE than Be
same shielding
greater nuclear charge
2e-
2e2e--
H
4+ 2e- 2e
4+
Be
Be
B
Li
3e- 5+
5+ 2e 3e
B
p-orbitals available
2p
2s
1s
Atomic number
First Ionization energy
He
n
H
C
Be
B
Li
2p
2s
1s
Atomic number
First Ionization energy
He
n
N
H
C
Be
B
Li
2p
2s
1s
Atomic number
First Ionization energy
He
n
N
•
H
C O
Be
Breaks the pattern because
removing an electron
gets to 1/2 filled p orbital
B
Li
2p
2s
1s
Atomic number
First Ionization energy
He
n
N
H
F
C O
Be
B
Li
2p
2s
1s
Atomic number
First Ionization energy
He
Ne
n
N
F
• Ne has a lower IE than He
H
C O
Be
• Both are full energy levels,
• Ne has more shielding
• Greater distance
B
Li
2p
2s
1s
Atomic number
First Ionization energy
He
Ne
n
N
F
• Na has a lower IE than Li
H
C O
Be
• Both are s1
• Na has more shielding
• Greater distance
B
3s
Li
2p
2s
Na
1s
Atomic number
First Ionization energy
He
n
H
Be has higher IE than Li
same shielding
greater nuclear charge
2e-
Be
2e1e-
2e-
3+
4+
Li
Be
Li
Atomic number
First Ionization energy
He
n
B has lower IE than Be
same shielding
greater nuclear charge
2e-
2e2e-
H
4+
Be
Be
B
Li
3e5+
B
p-orbitals available
2p
2s
1s
Atomic number
First Ionization energy
He
Ne
N
F
H
C O
Be
Na has a lower IE than
Li
Both are s1
Na has more shielding
Greater distance
B
Li
Na
Atomic number
He
Ne
Ar
First Ionization energy
Kr
H
Li
Na
K
Rb
Atomic number
5s
5p
4d
18
4s
He
4p
3d
18
Ne
Ar
3s
3p
8
First Ionization energy
2s
Kr
2p
8
H
1s
2
NUCLEUS
Li
Na
K
Rb
Atomic number
First Ionization
Energy Plot
5s
5p
4d
18
4s
4p
3d
18
3s
3p
8
2500
2s
He
2p
First ionization energy (kJ/mol)
8
Ne
2000
1s
F
1500
N
1000
C
Be
O
B
0
Na
5
Br
P
Mg
Li
Kr
NUCLEUS
Cl
H
500
2
Ar
10
Zn
S
Si
Al
Fe Ni
Ti
Cr
Ca
Co Cu
Mn
Sc V
As
Ge
Se
Sr
Ga
K
15
Rb
20
Atomic number
25
30
35
40
5
B
5+
Boron
2e-
2e3e-
B=
1s22s22p1
10.811
Isoelectronic
2e2e- 2e5+
5+
5+
5+ 2e 3e
B
>
2e-
B1+
5+ 2e- 2e-
n
B1+
= Be =
1s22s2
4+ 2e- 2e-
< Be
B1+ vs.
2e-
2e--
5+
5+ 2e 2e
B1+ >
2e-
1e
5+
5+ 2e 1e
B2+
n
B2+ = Li =1s22s1
5+ 2e- 1e-
3+ 2e- 1e-
< Li
B2+ vs.
2e2e-
5+
5+ 2e 1e
B2+ >
0e2e- 0e5+
5+
B3+
n
B3+ = He = 1s2
5+ 2e- 0e-
2+ 2e- 0e-
< He
B3+ vs.
5
B
5+
B=
1s22s22p1
10.811
Isoelectronic
Boron
5+ 2e- 3e-
B
5+ 2e- 2e-
>
5+ 2e- 2e-
B1+ >
5+ 2e- 1e-
B2+ >
B1+
5+ 2e- 1e-
B2+
5+ 2e- 0e-
B3+
5+ 2e- 2e-
n
B1+
= Be =
1s22s2
n
B2+ = Li =1s22s1
n
B3+ = He = 1s2
4+ 2e- 2e-
< Be
B1+ vs.
5+ 2e- 1e-
3+ 2e- 1e-
< Li
B2+ vs.
5+ 2e- 0e-
2+ 2e- 0e-
< He
B3+ vs.
16
S
32.066
16+
Sulfur
Isoelectronic
S = 1s22s22p63s23p4
16+ 2e- 8e- 6e-
S
16+ 2e- 8e- 7e-
<
16+ 2e- 8e- 7e-
S1-
S1-
16+ 2e- 8e- 8e-
<
S2-
n
S1- = Cl
1s22s22p63s23p5
n
S2- = Ar
1s22s22p63s23p6
16+ 2e- 8e- 7e-
S1-
17+ 2e- 8e- 7e-
>
vs.
16+ 2e- 8e- 8e-
S2-
Cl
18+ 2e- 8e- 8e-
>
vs.
Ar
Ionization Energies
• Energy is required to remove an electron from an atom to form a
cation.
• Ionization energy () is the amount of energy needed to remove an
electron from the gaseous atom E in its ground state:
E (g) + E+(g) + e-- energy required for reaction = .
• Ionization energy is always positive ( > 0).
• Larger values of mean that the electron is more tightly bound to the
atom and is harder to remove.
• Units for ionization energies are kilojoules/mole (kJ/mol) or electron
volts (eV) - 1 eV = 96.49 kJ/mol.
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Ionization Energies (in kilojoules per mole)
2nd
3rd
4th
Element
1st
H
1312.1
He
2372.5
5250.7
Li
520.3
7298.5
11815.6
Be
899.5
1752.2
14849.5
21007.6
B
800.7
2427.2
3660.0
25027.0
32828.3
C
1086.5
2352.8
4620.7
6223.0
37832.4
47279.4
Al
577.6
1816.7
2744.8
11577.5
14831.0
18377.9
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 190
5th
6th
Ionization Energies (kJ/mol)
2nd
3rd
4th
Element
1st
H
1312.1
He
2372.5
5250.7
Li
520.3
7298.5
11815.6
Be
899.5
1752.2
14849.5
21007.6
B
800.7
2427.2
3660.0
25027.0
32828.3
C
1086.5
2352.8
4620.7
6223.0
37832.4
47279.4
Al
577.6
1816.7
2744.8
11577.5
14831.0
18377.9
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 190
5th
6th
Ionization Energies (kJ/mol)
Element
1st
2nd
3rd
4th
5th
6th
Na
498
4560
6910
9540
13,400
16,600
Mg
736
1445
7730
10,600
13,600
18,000
Al
577
1815
2740
11,600
15,000
18,310
Si
787
1575
3220
4350
16,100
19,800
P
1063
1890
2905
4950
6270
21,200
S
1000
2260
3375
4565
6950
8490
Cl
1255
2295
3850
5160
6560
9360
Ar
1519
2665
3945
5770
7320
8780
Herron, Frank, Sarquis, Sarquis, Cchrader, Kulka, Chemistry 1996, Heath, page
Shaded area on table denotes core electrons.
Ionization Energies (kJ/mol)
Element
1st
2nd
3rd
4th
5th
6th
Na
498
4560
6910
9540
13,400
16,600
Mg
736
1445
7730
10,600
13,600
18,000
Al
577
1815
2740
11,600
15,000
18,310
Si
787
1575
3220
4350
16,100
19,800
P
1063
1890
2905
4950
6270
21,200
S
1000
2260
3375
4565
6950
8490
Cl
1255
2295
3850
5160
6560
9360
Ar
1519
2665
3945
5770
7320
8780
Herron, Frank, Sarquis, Sarquis, Cchrader, Kulka, Chemistry 1996, Heath, page
Shaded area on table denotes core electrons.
ionization energy: the energy required to remove
an e– from an atom
M + 1st I.E.
removes 1st e–
M1+ + e–
M + 2nd I.E.
M2+ + e–
M + 3rd I.E.
M3+ + e–
Each successive ionization requires
more energy than the previous one.
As we go , 1st I.E…. decreases.
(due to the shielding effect)
As we go
, 1st I.E…. increases.
Multiple Ionization Energies
Al+
Al
1st Ionization
energy
2nd Ionization
energy
Al2+
Al3+
3rd Ionization
energy
The second, third, and fourth ionization energies of aluminum are higher
than the first because the inner electrons are more tightly held by the nucleus.
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 190
Ionization Energies
• It takes more energy to remove the second electron from an atom
than the first, and so on.
• There are two reasons for this trend:
1. The second electron is being removed from a positively
charged species rather than a neutral one, so more energy is
required.
2. Removing the first electron reduces the repulsive forces
among the remaining electrons, so the attraction of the
remaining electrons to the nucleus is stronger.
• Energy required to remove electrons from a filled core is prohibitively
large and simply cannot be achieved in normal chemical reactions.
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Factors Affecting Ionization Energy
Nuclear Charge
The larger the nuclear charge, the greater the ionization energy.
Shielding effect
The greater the shielding effect, the less the ionization energy.
Radius
The greater the distance between the nucleus and the outer
electrons of an atom, the less the ionization energy.
Sublevel
An electron from a full or half-full sublevel requires additional
energy to be removed.
Smoot, Price, Smith, Chemistry A Modern Course 1987, page 189
Formation of Cation
sodium atom
Na
sodium ion
Na+
ee-
e-
e-
e-
e-
ee-
e-
11p+
ee-
loss of
one valence
electron
e-
e-
11p+
e-
e-
e-
e-
e-
e-
e-
e-
Formation of Anion
chlorine atom
Cl
e-
egain of
one valence
electron
ee-
e-
e-
chloride ion
Cl1e-
eee-
e-
e-
e-
e-
ee-
e-
17p+
17p+
e-
e-
e-
e-
ee-
e-
e-
e-
e-
e-
e-
e-
e-
e-
eee-
e-
Formation of Ionic Bond
chloride ion
Cl1-
sodium ion
Na+
e-
e-
ee-
e-
e-
e-
e-
e-
e-
e-
e-
11p+
e-
e-
e-
e-
e-
e-
17p+
e-
e-
e-
e-
e-
e-
ee-
e-
e-
Metallic Characteristic
metallic character increases
nonmetallic character increases
metallic character increases
nonmetallic character increases
Nuclear charge increases
Shielding increases
Atomic radius increases
Ionic size increases
Ionization energy decreases
Electronegativity decreases
Summary of Periodic Trends
Shielding is constant
Atomic radius decreases
Ionization energy increases
Electronegativity increases
Nuclear charge increases
1A
0
2A
Ionic size (cations)
decreases
3A 4A 5A 6A 7A
Ionic size (anions)
decreases
Modern Periodic Table
Essential Elements
H
Elements in organic matter
1
Major minerals
Li
Be
3
4
He
2
Trace elements
Na Mg
11
K
19
12
Ca Sc
20
Rb Sr
37
38
21
Y
39
Cs Ba La
55
56
57
Ti
V
22
23
B
C
N
O
F
Ne
5
6
7
8
9
10
Al
Si
P
S
Cl
Ar
13
14
15
16
17
18
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br
24
25
26
27
28
29
30
Zr Nb Mo Tc Ru Rh Pd Ag Cd
40
41
42
Hf
Ta
W
72
72
74
Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 748
43
44
Re Os
75
76
45
Ir
77
46
47
48
Pt Au Hg
78
79
80
31
In
49
Tl
81
32
33
34
Sn Sb Te
50
51
Pb Bi
82
83
52
Kr
35
36
I
Xe
53
54
Po At Rn
84
85
86
Trace Elements in Biological Systems
• Of the 100 known elements, 28 are known to be essential for the
growth of at least one biological species, and only 19 are essential
to humans.
• The following makes some elements essential:
1. The element must have some unique chemical property that
an organism can use to its advantage and without which it
cannot survive.
2. Adequate amounts of the element must be available in the
environment in an easily accessible form.
• Many of the elements essential to life are necessary in only small
amounts (trace elements).
Copyright 2007 Pearson Benjamin Cummings. All rights reserved.
Oxidation States of Elements
1
8
Groups
2
3
Li1+ Be2+
Na1+ Te2-
Al3+
K1+ Te2-
Zn2+ Ga3+
Rb1+ Te2-
Cs1+ Te2-
Ag1+
Transition metals form cations
with various charges.
In3+
4
5
6
7
O2-
F1-
S2-
Cl1-
Se2- Br1-
Te2-
I1-
Chemical Bonding
• Ionic
– Metal (cation) with non-metal (anion)
– Transfer of electron(s)
– Strong bond…high melting point
• Covalent
– Non-metal with non-metal
– Sharing of electron(s)
• Non-polar (equal distribution of electrons)
• Polar (uneven electron distribution)
– Weak bonds…low melting points
• Single, double and triple bonds
• Metallic (nuclei in a “sea” of shared electrons)
First Four Energy Levels
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 334
Modern Atomic Structure
Sublevel designation
n=4
4s
4p
n=3
n=2
3s
4d
3p
2s
An orbital for a hydrogen
atom. The intensity of the
dots shows that the electron
spends more time closer to
the nucleus.
4f
3d
2p
1s
n=1
The first four principal energy
levels in the hydrogen atom.
Each level is assigned a
principal quantum number n.
Hein, Arena, Foundations of College Chemistry, 2000, page 202
The types of orbitals on each
of the first four principal
energy levels.
Autobiography of an Element
I am Promethium, Pm for short. I was named after Prometheus, who
according to Greek mythology, brought fire to man. I'm a member of the
Lanthanide (rare earth) elements. My family name is derived from the Greek
lanthum, meaning “to escape notice”.
You may not have noticed me around before, as I have no naturally
occurring isotopes. True to family tradition, I managed to avoid positive
identification until 1965. O. Ermetsa first isolated 350 mg 147Pm from 6000
tons apatite. Once discovered, I was immediately put to work. Large quantities
of 147Pm salts (luminesce pale blue or green) are used in luminescent paint
for watch dials. Another job I've held is as a part in a beta-voltaic battery.
You may think there is not enough of me to go around. However, everyday,
my cousin 147Sm transforms into Promethium by radioactive decay (at a rate of
0.07%/day). Also, I'm a rare earth fission product of uranium. Please get to
know me. I'll be around for awhile with 147Pm half-life of 2.5 years and 145Pm
half-life of 30 years.
Neon Advertisement
Oxygen
Fluorine
Chlorine
O
F
Cl
15.999
18.998
35.453
Hydrogen
Neon
Argon
Ar
H
1.0079
$10,895
39.948
Helium
Xenon
Krypton
He Xe Kr
4.0026
131.30
83.800
*Neon Highline Sedan, shown: $13,770 nicely equipped. MSRPs include destination, exclude tax. *Achieved with premium unleaded fuel.
When utilizing the Ideal Gas Equation, PV = nRT, remember that temperature is measured in Kelvins.
Exception!
Two exceptions to the simple –ide ending are the diatomic oxide ions,
O22- and O21-.
O22- is called peroxide
Note the differences.
O21- is called superoxide.
barium oxide
barium peroxide
BaO
__________
BaO2
__________
sodium oxide
sodium peroxide
Na2O
__________
Na2O2
__________
potassium oxide
potassium superoxide
K2O
__________
KO2
__________
Ba2+
Na1+
Do Not Reduce to lowest terms!
K1+
Resources - Periodic Table
Objectives
Episode 7 – The Periodic Table
Worksheet - vocabulary
General Chemistry PP
Activity - aliens cards: A B key
Activity - coloring periodic table
Worksheet - periodic table paragraph
Worksheet - ionization energies
Lab - periodic trends database
Project - element brochure example timeline
Worksheet - periodic table textbook questions
Worksheet - textbook questions (general)
Outline (general)





