Elements, Compounds, Mixtures, Law of
advertisement
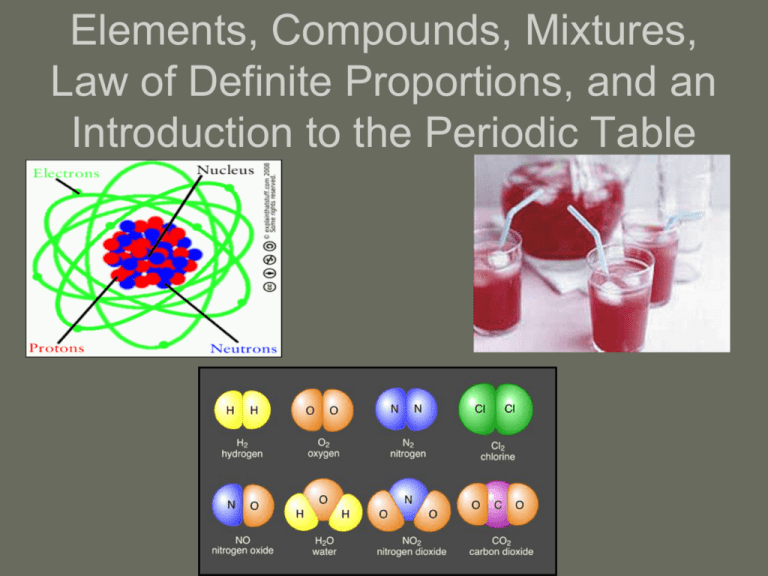
Elements, Compounds, Mixtures, Law of Definite Proportions, and an Introduction to the Periodic Table Elements and Compounds Element: A pure substance that CANNOT be separated into a simpler substance by physical or chemical means. GOLD COPPER There are 91 naturally occurring elements on earth Approximately 24 synthetic elements have been identified Symbols of Elements The symbols of elements are regulated by the International Union of Pure and Applied Chemistry (IUPAC), and they are derived in several ways. Many elemental symbols are simply the capitalized letter of the first letter of the English name of the element: C is for Carbon N is for Nitrogen If two elements start with the same first letter, then a second lowercase letter will be added to differentiate them: Ca is for Calcium Ni is for Nickel Derivation of Element Names Many elements are named after their Latin derivation: Lead which is “plumbus” in Latin is Pb Gold which is “aurum” in Latin is Au Potassium which is “kalium” in Latin is K Other elements are named to honor a famous scientist: Es for Einsteinium Cm for Curium Rf for Ruthifordium Still other elements are named for the country in which they were discovered: Fr for Francium Am for Americium Compounds Combinations of two or more elements that are combined chemically: H20 NaCl C6H12O6 Al2O3 Fe2O3 Matter Mixture Homogeneous Substance Heterogeneous Solution Colloid Suspension Element Compound Elements and Compounds Similarities 1. Both are substances Differences 1. Compounds are substances that can be broken down into elements 2. Both have physical and 2. Elements are chemical properties substances that cannot be broken down Mixtures of Matter Characteristics of a mixture: a.) A combination of two or more pure substances in which each individual substance that makes up the mixture retains its individual properties b.) Properties are largely those of its component parts; conversely when the individual substances combine new properties are NOT created c.) It is a blend of two or more substances d.) It does not have a constant composition e.) A mixture is a blend of substances; not a chemical bonding of substances f.) They can be separated by physical means Types of Mixtures Heterogeneous: Individual substances still remain distinct A mixture that may have many phases Homogenous: A mixture is the same throughout A mixture that contains a single phase ALSO CALLED A SOLUTION! Same or Different? a.) substance and a pure substance b.) heterogeneous mixture and solution c.) substance and mixture d.) homogenous mixture and solution Law of Definite Proportions A compound is always composed of the same elements in the same proportions by mass, regardless of the amount. % by mass = mass of element x 100 mass of compound OR (in other words) % by mass = part whole x 100 Mass of H20: H: 2(1) = 2 amu O: 1(16) = 16 amu 2 amu from H + 16 amu from O = 18 amu for H20 % of H in H2O: (2 amu/18 amu) x 100 = 11.1% Hydrogen % of O in H2O: (16 amu/18 amu) x 100 = 88.9% Oxygen Sample Problem 1: Suppose you break a compound down into its elements and find that 25.00 g of the compound is composed of 6.77 g of tin and 18.23 g of bromine. The percent by mass of tin in the compound can be determined as follows: Percent by mass of tin = mass of tin mass of compound x 100 Percent by mass of tin = 6.77 g x 100 = 27.1% tin 25.00 g Follow the procedure described above to determine the percent by mass of bromine in the compound discussed above. What is the sum of the percents of the two elements? Sample Problem 2: A 134.50 g sample of aspirin is made up of 6.03 g of hydrogen, 80.70 g of carbon, and 47.77 g of oxygen. What is the percent by mass of each element in aspirin? Sample Problem 3: A 2.89 g sample of sulfur reacts with 5.72 g of copper to form a black compound. What is the percentage composition of the compound? Practice I 1. If hydrogen composes 11% of a 100 gram sample of water, what is the mass of the hydrogen in the sample? 2. If one gram of hydrogen reacts completely with 19.9 g of fluorine, what is the % by mass of hydrogen in the compound that is formed? Practice Continued 3. If 3% of a 100 g HCl solution is hydrogen, what is the mass of the chlorine in the sample? 4. A 78.0 g sample of an unknown compound contains 12.4 g of hydrogen. What is the percent by mass of hydrogen in the compound? Practice Continued 5. If 40% of a 300 g NaCl solution is sodium, what is the mass of the sodium present in the sample? The Periodic Table The Periodic Table – A chart that organizes the elements based on their similarities and proton number. The original Periodic Table was developed by a Russian named Dmetri Mendeleev in 1869 Subatomic Particles: Protons, Neutrons, Electrons Upon looking at the periodic table, we find many clues as to how to find information about each individual element. Finding Protons, Neutrons, Electrons The Number of Protons (p+) is... the number of p+ in an atom of an element. In this example, krypton's atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. The Number of Electrons (e-) is... by definition, atoms have no overall electrical charge. That means that there must be a balance between the positively charged protons and the negatively charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. The Number of Neutrons (no) is... # of Neutrons = Mass Number - Atomic Number – to find the mass number, all you need to do is round the atomic weight to the nearest whole number. In our example, krypton's mass number is 84 amu since its atomic weight, 83.80, rounds up to 84 amu. – The mass number is a count of the number of particles in an atom's nucleus. Remember that the nucleus is made up of protons and neutrons. So, we can write: Mass Number = (Number of Protons) + (Number of Neutrons) For Krypton, this equation becomes: 84 = (Number of Protons) + (Number of Neutrons) States of Matter and the Periodic Table Organization of the Periodic Table Properties of the Periodic Table





