1
CHEMICAL
EQUILIBRIUM
Chapter 13
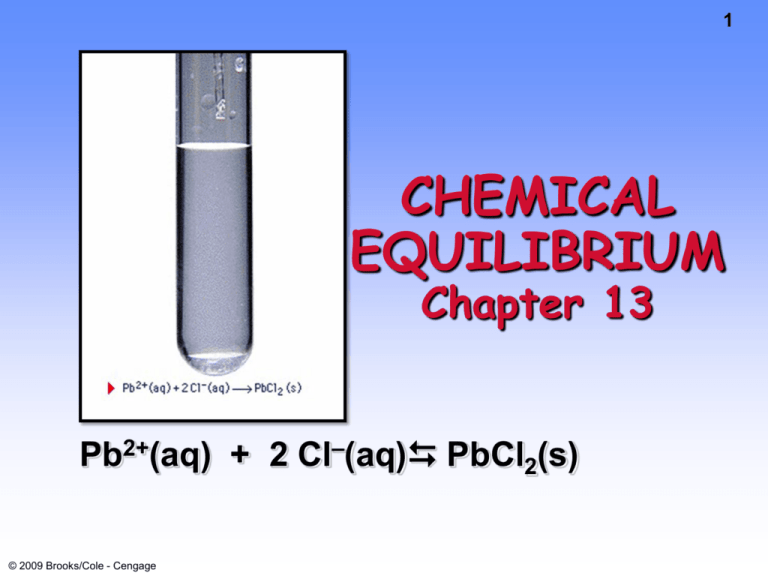
Pb2+(aq) + 2 Cl–(aq) PbCl2(s)
© 2009 Brooks/Cole - Cengage
2
Objectives
• Briefly review what we know of equilibrium
• Define the Equilibrium Constant (Keq) and
Reaction Quotient (Q)
• Determining the Keq
• Using Keq in calculation
• Equations and Manipulating Keq
• Disturbing a Chemical Equilibrium and Le
Châtelier’s Principle
© 2009 Brooks/Cole - Cengage
3
Ch. 13.1 – The Equilibrium Condition
Equilibrium systems are
• DYNAMIC (in constant
motion)
• REVERSIBLE
• can be approached from
either direction
Pink to blue
Co(H2O)6Cl2 Co(H2O)4Cl2 + 2 H2O
Blue to pink
Co(H2O)4Cl2 + 2 H2O Co(H2O)6Cl2
© 2009 Brooks/Cole - Cengage
4
Chemical Equilibrium
Fe3+ + SCN- FeSCN2+
+
Fe(H2O)63+ + SCN- Fe(SCN)(H2O)52+ + H2O
© 2009 Brooks/Cole - Cengage
Chemical Equilibrium
Fe3+ + SCN- FeSCN2+
• After a period of time, the concentrations of
reactants and products are constant.
• The forward and reverse reactions continue
after equilibrium is attained.
© 2009 Brooks/Cole - Cengage
5
6
Examples of
Chemical Equilibria
Phase changes such as
H2O(s) H2O(liq)
© 2009 Brooks/Cole - Cengage
7
Examples
of Chemical
Equilibria
Formation of
stalactites and stalagmites
CaCO3(s) + H2O(liq) + CO2(g)
Ca2+(aq) + 2 HCO3-(aq)
© 2009 Brooks/Cole - Cengage
8
Chemical
Equilibria
CaCO3(s) + H2O(liq) + CO2(g)
Ca2+(aq) + 2 HCO3-(aq)
At a given T and P of CO2, [Ca2+] and [HCO3-]
can be found from the EQUILIBRIUM
CONSTANT, Keq
© 2009 Brooks/Cole - Cengage
9
Ch. 13.2 – The Equilibrium Constant
N2(g) + 3H2(g) 2NH3(g)
Reactant conc. declines and then
becomes constant at equilibrium
Product conc. increases and then
becomes constant at equilibrium
© 2009 Brooks/Cole - Cengage
10
The Equilibrium Constant
• Concentrations reach levels where
the rate of the forward reaction
equals the rate of the reverse
reaction.
Rateforward = Ratebackward
© 2009 Brooks/Cole - Cengage
11
The Equilibrium Constant
© 2009 Brooks/Cole - Cengage
THE EQUILIBRIUM CONSTANT
For any type of chemical equilibrium of the
type
aA + bBcC + dD
the following is a CONSTANT at a given T
If K (aka Kc) is known, then we can
predict concs. of products or reactants.
© 2009 Brooks/Cole - Cengage
12
Practice 1: Write the equilibrium
expression (K) for the following
reactions:
a)
b)
c)
d)
e)
2O3(g) 3O2(g)
2NO(g) + Cl2(g) 2NOCl(g)
Ag+(aq) + 2NH3(aq) Ag(NH3)2+(aq)
H2(g) + I2(g) 2HI(g)
Cd2+(aq) + 4Br-(aq) CdBr42-(aq)
© 2009 Brooks/Cole - Cengage
13
Practice 2
Consider an equilibrium mixture in a closed
vessel reacting according to the equation:
H2O(g) + CO(g) H2(g) + CO2(g)
You add more H2O(g) to the flask. How does
the concentration of each chemical compare
to its original concentration after
equilibrium is reestablished? Justify your
answer.
© 2009 Brooks/Cole - Cengage
14
Determining K
When you know the equilibrium
concentrations of the reactants and products,
the K can be determined by substituting the
values into the equilibrium constant
expression.
Example: In an experiment of the reaction at
600°C:
2SO2(g) + O2(g) 2SO3(g)
The equilibrium concentrations are:
[SO2] = 1.50 M
[O2] = 1.25 M
[SO3] = 3.50 M
© 2009 Brooks/Cole - Cengage
15
16
Determining K
In an experiment of the reaction at 600°C,
2SO2(g) + O2(g) 2SO3(g)
Determine K:
𝑺𝑶𝟑 𝟐
𝟑. 𝟓𝟎 𝟐
𝐊=
=
= 𝟒. 𝟑𝟔
𝟐
𝟐
𝑺𝑶𝟐 [𝑶𝟐]
𝟏. 𝟓𝟎 (𝟏. 𝟐𝟓)
© 2009 Brooks/Cole - Cengage
Writing and Manipulating K Expressions
Changing coefficients
2SO2(g) + O2(g) 2SO3(g)
4SO2(g) + 2O2(g) 4SO3(g)
Knew =
[𝑺𝑶𝟑]𝟒
𝑺𝑶𝟐 𝟒 𝑶𝟐
K=
Knew = (4.36)2 = 19.0
© 2009 Brooks/Cole - Cengage
[𝑺𝑶𝟑]𝟐
𝑺𝑶𝟐 𝟐 𝑶𝟐
Knew =
𝟐
=
𝑲
𝟐
𝒐𝒍𝒅
17
[𝑺𝑶𝟑]𝟒
𝑺𝑶𝟐 𝟒 𝑶𝟐
Note:
Knew can also
be noted as
K’ (K prime)
𝟐
18
Writing and Manipulating
K Expressions
Changing direction
2SO2(g) + O2(g) 2SO3(g)
2SO3(g) 2SO2(g) + O2(g)
Knew =
1
[𝑺𝑶𝟑]𝟐
𝟐
𝑺𝑶𝟐 𝑶𝟐
=
Knew =
𝑺𝑶𝟐 𝟐 𝑶𝟐
𝑺𝑶𝟑 𝟐
Knew = 1/4.36 = 0.229
© 2009 Brooks/Cole - Cengage
K=
[𝑺𝑶𝟑]𝟐
𝑺𝑶𝟐 𝟐 𝑶𝟐
𝑺𝑶𝟐 𝟐 𝑶𝟐
𝑺𝑶𝟑 𝟐
= 1/Kold
Writing and Manipulating K Expressions
How do these manipulations affect the
equilibrium concentration values?
• Find the equilibrium [O2] using the equation
4SO2(g) + 2O2(g) 4SO3(g) K= 19.0
if [SO2] = 1.50 M
and [SO3] = 3.50 M
• [O2] =
𝑆𝑂3 4
𝑆𝑂2 4 𝐾
=
3.50 4
1.50 4 ∗19.0
= 1.25 M (Same as before!)
© 2009 Brooks/Cole - Cengage
19
20
Writing and Manipulating K Expressions
• K always has the same value at a given
temperature regardless of the amounts
of reactants or products that are
present initially.
• For a reaction, at a given temperature,
there are many equilibrium positions
but only one equilibrium constant, K.
– Equilibrium position is a set of
equilibrium concentrations.
© 2009 Brooks/Cole - Cengage
21
Writing and Manipulating K Expressions
Equilibrium position is a set of
equilibrium concentrations.
2SO2(g) + O2(g) 2SO3(g)
Experiment 2
Equil [ ]’s 0.590 M 0.0450 M 0.260 M
𝐾=
© 2009 Brooks/Cole - Cengage
2
0.260
= 4.32
2
0.590 (0.0450)
22
Manipulating K Practice Problem
Use the data below to find the
equilibrium concentration of SO2 if the
equilibrium concentrations of SO3 and O2
are:
[SO3] = 2.25 M
[O2] = 1.50 M
Use a reaction and K from at least two
different equations. See slides 16 to 18.
© 2009 Brooks/Cole - Cengage
Ch. 13.3 – Equilibrium Expressions
Involving Pressures
Concentration Units
We have been writing K in terms of mol/L.
These are designated by K or Kc
But with gases, P = (n/V)·RT = conc · RT
P is proportional to concentration, so we can write K
in terms of P. These are designated by Kp.
Kc and Kp may or may not be the same.
© 2009 Brooks/Cole - Cengage
23
Convert between Kc and Kp
General gas reaction aA + bB
cC + dD
PC c PD d
ni
K p a b , but Pi ( ) RT or Pi M i RT so
PA PB
V
(M C RT)c (M D RT)d
[M C ]c [M D ]d RT (c+d)
Kp =
or K p
a
b
(M A RT) (M B RT)
[M A ]a [M B ]b RT (a+b)
Kp=Kc(RT)(c+d-a-b)
R=0.0821L-atm/mol•K
Kc
Also thought of as Δn, or
change in number of moles of
gas.
© 2009 Brooks/Cole - Cengage
24
Writing and Manipulating
K Expressions
K using concentration and pressure units
Kp = Kc (RT)∆n
For
S(s) + O2(g) SO2(g)
∆n = 0 and Kp = Kc
For SO2(g) + 1/2 O2(g) SO3(g)
∆n = –1/2 and Kp = Kc(RT)–1/2
© 2009 Brooks/Cole - Cengage
25
26
Example with Kp
At 500 K, the following equilibrium is
established:
2NO(g) + Cl2(g) ⇌ 2NOCl(g).
0.095 0.171
0.28
An equilibrium mixture of the three gases has
a partial pressures of 0.095 atm, 0.171 atm and
0.28 atm for NO, Cl2 and NOCl, respectively.
Calculate the Kp for this reaction at 500 K.
Kp =
© 2009 Brooks/Cole - Cengage
𝑷𝟐 𝑵𝑶
𝟐
𝟐
𝑷 𝑵𝑶 𝑷𝑪𝒍
=
𝟐
(𝟎.𝟐𝟖)𝟐
(𝟎.𝟎𝟗𝟓)𝟐 (𝟎.𝟏𝟕𝟏)
= 51
Practice with Kp
27
A mixture of NO, H2, and H2O are placed in a 1.0 L
vessel at 400K. The following equilibrium
pressures are established:
2NO(g) + 2H2(g) ⇌ N2(g) + 2H2O(g).
Equil (atm) 1.53
0.300
0.465
3.39
Find Kp. Convert pressures to molarity and
determine Kc. Compare with Kp to Kc conversion
© 2009 Brooks/Cole - Cengage
28
Ch. 13.4 – Heterogeneous
Equilibria
Pure solids and liquids
NEVER appear in
equilibrium
expressions.
S(s) + O2(g)
SO2(g)
K=
© 2009 Brooks/Cole - Cengage
[SO2 ]
[O2 ]
29
Heterogeneous Equilibria
Pure solids and liquids NEVER
appear in equilibrium
expressions.
NH3(aq) + H2O(liq)
NH4+(aq) + OH-(aq)
K=
© 2009 Brooks/Cole - Cengage
[NH4+ ][OH- ]
[NH3 ]
30
What Are the Equilibrium Expressions
for These Reactions?
SnO2(s) + 2CO(g) Sn(s) + 2CO2(g)
CaCO3(s) CaO(s) + CO2(g)
Zn(s) + Cu2+(aq) Cu(s) + Zn2+(aq)
© 2009 Brooks/Cole - Cengage
31
Writing and Manipulating K Expressions
When adding equations for reactions
S(s) + O2(g) SO2(g)
K1 =
SO2(g) + 1/2 O2(g) SO3(g)
The net equation is
K2 =
[SO2 ]
[O2 ]
[SO3 ]
[SO2 ][O2 ]1/2
S(s) + 3/2 O2(g) SO3(g)
And then multiply the individual K’s:
Knet = K1 x K2
© 2009 Brooks/Cole - Cengage
Knet =
[SO3 ]
[O2 ]3/2
Manipulating Equilibrium Constants
Practice
32
Determine the Keq for the following reaction:
2NH3(g) + 3I2(g) 6HI(g) + N2(g)
3*(H2(g) + I2(g) 2HI(g))
-1(N2(g) + 3H2(g) 2NH3(g))
K1 = (5.40 x 101)3
K2 = (1.04 x 10-4)-1
3H2(g) + 3I2(g) 6HI(g)
K’1 = 1.57 x 105
2NH3(g) N2(g) + 3H2(g) K’2 = 9.62 x 103
2NH3(g) + 3I2(g) 6HI(g) + N2(g)
Keq = K’1 * K’2 = 1.57x105(9.62x103) = 1.51 x 109
© 2009 Brooks/Cole - Cengage
Ch. 13.5 – Applications of
the Equilibrium Constant
1.
Can tell if a reaction is product-favored
or reactant-favored.
For N2(g) + 3 H2(g) 2 NH3(g)
Kc =
[NH3 ]2
[N2 ][H2 ]3
= 3.5 x 108
A large value of K means the concentration of
products is much greater than that of
reactants at equilibrium.
The reaction is strongly
product-favored.
© 2009 Brooks/Cole - Cengage
33
The Meaning of K
34
For AgCl(s)
Ag+(aq) + Cl-(aq)
Kc = [Ag+] [Cl-] = 1.8 x 10-5
Conc. of products is much
less than that of reactants
Ag+(aq) + Cl-(aq)
at equilibrium.
The reaction with small K is AgCl(s)
is product-favored.
strongly
reactant-favored.
© 2009 Brooks/Cole - Cengage
35
Product- or Reactant Favored
Product-favored
K>1
© 2009 Brooks/Cole - Cengage
Reactant-favored
K<1
The Reaction Quotient, Q
In general, ALL reacting chemical systems are
characterized by their REACTION
QUOTIENT, Q.
aA + bBcC + dD
If Q = K, then system is at equilibrium.
© 2009 Brooks/Cole - Cengage
36
Reaction Quotient &
Equilibrium Constant
At any point in the reaction
H2 + I2 2 HI
[HI]2
Q = reaction quotient =
[H2 ][I2 ]
© 2009 Brooks/Cole - Cengage
37
Reaction Quotient &
Equilibrium Constant
Equilibrium achieved
In the equilibrium region
[HI]2
= 55.3 = K
[H2 ][I2 ]
K = equilibrium constant
© 2009 Brooks/Cole - Cengage
38
The Meaning of K
2. Can tell if a reaction is at equilibrium. If not,
which way it moves to approach equilibrium.
n-butane
H H H H
H—C—C—C—C—H
H H H H
K =
© 2009 Brooks/Cole - Cengage
[iso]
[n]
iso-butane
H H H
H—C—C—C—H
H
H
H C H
H
= 2.5
39
The Meaning of K
n-butane
H H H H
H—C—C—C—C—H
H H H H
K =
[iso]
[n]
iso-butane
H H H
H—C—C—C—H
H
H
H C H
H
= 2.5
If [iso] = 0.35 M and [n] = 0.15 M, are
you at equilibrium?
If not, which way does the reaction
“shift” to approach equilibrium?
© 2009 Brooks/Cole - Cengage
40
If Q = K,
the system is at equilibrium.
© 2009 Brooks/Cole - Cengage
41
If Q > K,
there is too much product and the equilibrium
shifts to the left.
© 2009 Brooks/Cole - Cengage
42
If Q < K,
there is too much reactant, and the equilibrium
shifts to the right.
© 2009 Brooks/Cole - Cengage
43
The Meaning of K
All reacting chemical systems are
characterized by their REACTION
QUOTIENT, Q.
product concentrations
Q=
reactant concentrations
If Q = K, then system is at equilibrium.
conc. of iso
0.35
Q=
=
= 2.3
conc. of n
0.15
Q (2.33) < K (2.5)
Reaction is NOT at equilibrium, so [iso] must
become ________ and [n] must
____________.
© 2009 Brooks/Cole - Cengage
44
Practice Problem
45
Ethyl acetate is synthesized in a nonreacting solvent
(not water) according to the reaction:
CH3CO2H + C2H5OH CH3CO2C2H5 + H2O K = 2.2
acetic acid ethanol
ethyl acetate
In the following mixtures, will the concentration of H2O
increase, decrease or remain the same as equilibrium is
established?
a) [CH3CO2C2H5]=0.22 M; [H2O]=0.10 M,
[CH3CO2H]= 0.010 M, [C2H5OH]=0.010 M
b) [CH3CO2C2H5]=0.22 M; [H2O]=0.0020 M,
[CH3CO2H]= 0.0020 M, [C2H5OH]=0.10 M
c) [CH3CO2C2H5]=0.88 M; [H2O]=0.12 M,
[CH3CO2H]= 0.044 M, [C2H5OH]=6.0 M
© 2009 Brooks/Cole - Cengage
Determining K
More typically, you will have to determine the
equilibrium concentrations:
2 NOCl(g) 2 NO(g) + Cl2(g)
Place 2.00 mol of NOCl is a 1.00 L flask. At
equilibrium you find 0.66 mol/L of NO.
Calculate K.
Solution
Set of an “ICE” table of concentrations
[NOCl]
[NO]
[Cl2]
Initial
2.00
0
0
Change
Equilibrium
0.66
© 2009 Brooks/Cole - Cengage
46
Determining K
2 NOCl(g) 2 NO(g) + Cl2(g)
Place 2.00 mol of NOCl is a 1.00 L flask. At
equilibrium you find 0.66 mol/L of NO.
Calculate K.
Solution
Set of an “ICE” table of concentrations
[NOCl]
[NO]
[Cl2]
Initial
2.00
0
0
Change
-0.66
+0.66
+0.33
Equilibrium
1.34
0.66
0.33
© 2009 Brooks/Cole - Cengage
47
Determining K
2 NOCl(g) 2 NO(g) + Cl2(g)
[NOCl]
[NO]
[Cl2]
Initial
2.00
0
0
Change
-0.66
+0.66
+0.33
Equilibrium
1.34
0.66
0.33
K=
K=
[NO]2 [Cl2 ]
2
[NOCl]
© 2009 Brooks/Cole - Cengage
[NO]2 [Cl2 ]
[NOCl]2
=
(0.66)2 (0.33)
2
(1.34)
= 0.080
48
Determine K Practice
A solution of 0.050 M diiodocyclohexane, C6H10I2 is
prepared in CCl4. When the solution has come to
equilibrium, the concentration of I2 is 0.035 M.
C6H10I2(sol) C6H10(sol) + I2(sol)
1) Determine the equilibrium concentrations of C6H10I2
and C6H10 at equilibrium.
2) Calculate the equilibrium constant, Kc.
© 2009 Brooks/Cole - Cengage
49
Ch. 13.6 – Solving Equilibrium
Problems
PROBLEM: Place 1.00 mol each of H2 and I2 in
a 1.00 L flask. Calc. equilibrium
concentrations.
H2(g) + I2(g) 2 HI(g)
[HI]2
Kc =
= 55.3
[H2 ][I2 ]
© 2009 Brooks/Cole - Cengage
50
H2(g) + I2(g) 2 HI(g)
Kc = 55.3
Step 1. Set up ICE table to define
EQUILIBRIUM concentrations.
Initial
Change
Equilib
© 2009 Brooks/Cole - Cengage
[H2]
[I2]
[HI]
1.00
1.00
0
51
H2(g) + I2(g) 2 HI(g)
Kc = 55.3
Step 1. Set up ICE table to define
EQUILIBRIUM concentrations.
[H2]
[I2]
[HI]
Initial
1.00
1.00
0
Change
-x
-x
+2x
Equilib
1.00-x
1.00-x
2x
where x is defined as am’t of H2 and I2
consumed on approaching equilibrium.
© 2009 Brooks/Cole - Cengage
52
H2(g) + I2(g) 2 HI(g)
Kc = 55.3
Step 2. Put equilibrium concentrations
into Kc expression.
[2x]2
Kc =
= 55.3
[1.00-x][1.00-x]
© 2009 Brooks/Cole - Cengage
53
H2(g) + I2(g) 2 HI(g)
Kc = 55.3
Step 3. Solve Kc expression - take
square root of both sides.
[2x]2
Kc =
= 55.3
[1.00-x][1.00-x]
2x
7.44 =
1.00-x
x = 0.79
Therefore, at equilibrium
[H2] = [I2] = 1.00 - x = 0.21 M
[HI] = 2x = 1.58 M
© 2009 Brooks/Cole - Cengage
54
55
Practice Problem
Consider the following reaction at 600ºC
2SO2(g) + O2(g) 2SO3(g)
• In a certain experiment 2.00 mol of SO2, 1.50
mol of O2 and 3.00 mol of SO3 were placed in
a 1.00 L flask. At equilibrium 3.50 moles of
SO3 were found to be present.
• Calculate the equilibrium concentrations of
O2 and SO2 and Kc.
© 2009 Brooks/Cole - Cengage
56
Practice Problem #1
2SO2(g) + O2(g) 2SO3(g)
Initial
Change
Equilibrium
[SO2]
2.00
-0.50
1.50
[O2]
1.50
-0.25
1.25
2
3.50
__
Kc =
=4.36
2
1.25(1.50)
© 2009 Brooks/Cole - Cengage
[SO3]
3.00
+0.50
3.50
57
Nitrogen Dioxide
Equilibrium
N2O4(g) 2 NO2(g)
© 2009 Brooks/Cole - Cengage
58
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
Kc =
[NO2 ]2
[N2O4 ]
= 0.0059 at 298 K
If initial concentration of N2O4 is 0.50 M, what are
the equilibrium concentrations?
Step 1. Set up an ICE table
[N2O4]
[NO2]
Initial
0.50
0
Change
Equilib
© 2009 Brooks/Cole - Cengage
59
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
Kc =
[NO2 ]2
[N2O4 ]
= 0.0059 at 298 K
If initial concentration of N2O4 is 0.50 M, what are
the equilibrium concentrations?
Step 1. Set up an ICE table
[N2O4]
[NO2]
Initial
0.50
0
Change
-x
+2x
Equilib
0.50 - x
2x
© 2009 Brooks/Cole - Cengage
60
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
Step 2. Substitute into Kc expression and solve.
[NO2 ]2
(2x)2
Kc = 0.0059 =
=
[N2O4 ] (0.50 - x)
Rearrange:
0.0059 (0.50 - x) = 4x2
0.0029 - 0.0059x = 4x2
4x2 + 0.0059x - 0.0029 = 0
This is a QUADRATIC EQUATION
ax2 + bx + c = 0
a = 4
b = 0.0059
c = -0.0029
© 2009 Brooks/Cole - Cengage
61
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
Solve the quadratic equation for x.
ax2 + bx + c = 0
a = 4
b = 0.0059
c = -0.0029
-b ± b2 - 4ac
x=
2a
-0.0059 ± (0.0059)2 - 4(4)(-0.0029)
x=
2(4)
x = -0.00074 ± 1/8(0.046)1/2 = -0.00074 ± 0.027
© 2009 Brooks/Cole - Cengage
62
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
-0.0059 ± (0.0059)2 - 4(4)(-0.0029)
x=
2(4)
x = -0.00074 ± 1/8(0.046)1/2 = -0.00074 ± 0.027
x = 0.026 or -0.028
But a negative value is not reasonable.
Conclusion: x = 0.026 M
[N2O4] = 0.50 - x = 0.47 M
[NO2] = 2x = 0.052 M
© 2009 Brooks/Cole - Cengage
63
Solving Quadratic
Equations
• Recommend you
solve the equation
exactly on a
calculator or use
the quadratic
equation when
Kc<<1. Otherwise,
assume x is small
relative to initial
conc (C>100*K)
© 2009 Brooks/Cole - Cengage
64
Solving Quadratic Equations
• Molecular Iodine can dissociate into atomic iodine as
shown by the reaction:
I2(g) ⇌ 2I(g)
The Keq for this reaction is:
Keq = [I]2 = 5.6 x 10-12
[I2]
• If the initial concentration of I2 is 0.45 M,
what are the equilibrium
concentrations?
© 2009 Brooks/Cole - Cengage
65
Example
I2(g) 2I(g)
Initial
Change
Equilibrium
[I2]
0.45
-x
0.45 – x
(2x)2
Keq =
(0.45 - x)
[I]
0
2x
2x
__
=5.6 x 10-12
X is going to be very small as
compared to 0.45 (0.45>>100*5.6x10-12)
© 2009 Brooks/Cole - Cengage
66
Example
I2(g) 2I(g)
Initial
Change
Equilibrium
[I2]
0.45
-x
0.45 – x
[I]
0
2x
2x
Which means we can ignore x in the
denominator and rewrite as:
2
(2x)
__
Keq =
=5.6 x 10-12
(0.45)
x = 7.9 x 10-7
So [I] = 2x = 2(7.9x10-7) or 1.6x10-6 M
© 2009 Brooks/Cole - Cengage
67
Practice Problem #2
At 35°C, K = 1.6 x 10-5 for the reaction
2NOCl(g) 2NO(g) + Cl2(g)
Calculate the concentration of all species at
equilibrium for each of the following initial
mixtures:
a) 2.0 mol pure NOCl in a 2.0 L flask
b) 1.0 mol of NOCl and 1.0 mol NO in a 1.0 L
flask
c) 2.0 mol NOCl and 1.0 mol Cl2 in a 1.0 L flask.
© 2009 Brooks/Cole - Cengage
Ch. 13.7 – Le Châtelier’s
Principle
• Temperature, catalysts, and changes in
concentration affect equilibria.
• The outcome is governed by LE
CHATELIER’S PRINCIPLE
• “...if a system at equilibrium is
disturbed, the system tends to shift its
equilibrium position to counter the
effect of the disturbance.”
© 2009 Brooks/Cole - Cengage
68
69
EQUILIBRIUM AND
EXTERNAL EFFECTS
Henri Le Châtelier
1850-1936
Studied mining
engineering.
Interested in glass
and ceramics.
© 2009 Brooks/Cole - Cengage
70
EQUILIBRIUM AND EXTERNAL EFFECTS
• Temperature change
change in K
• Consider the fizz in a soft drink
CO2(aq) + HEAT CO2(g) + H2O(liq)
• K = P (CO2) / [CO2]
• Increase T. What happens to equilibrium
position? To value of K?
• K increases as T goes up because P(CO2)
increases and [CO2] decreases.
• Decrease T. Now what?
• Equilibrium shifts left and K decreases.
© 2009 Brooks/Cole - Cengage
71
Temperature Effects
on Equilibrium
N2O4 (colorless) + heat
2 NO2 (brown)
∆Ho = + 57.2 kJ
Kc =
[NO2 ]2
[N2O4 ]
Kc (273 K) = 0.00077
Kc (298 K) = 0.0059
© 2009 Brooks/Cole - Cengage
72
Temperature Effects on
Equilibrium
© 2009 Brooks/Cole - Cengage
73
EQUILIBRIUM AND EXTERNAL EFFECTS
Catalytic exhaust system
• Add catalyst
no change in K
• A catalyst only affects the RATE of
approach to equilibrium.
© 2009 Brooks/Cole - Cengage
74
EQUILIBRIUM AND EXTERNAL EFFECTS
• Concentration changes
–no change in K
–only the equilibrium
composition changes.
© 2009 Brooks/Cole - Cengage
75
Le Chatelier’s Principle
Adding a “reactant” to a chemical
system.
© 2009 Brooks/Cole - Cengage
76
Le Chatelier’s Principle
Removing a “reactant” from a chemical
system.
© 2009 Brooks/Cole - Cengage
77
Le Chatelier’s Principle
Adding a “product” to a chemical
system.
© 2009 Brooks/Cole - Cengage
78
Le Chatelier’s Principle
Removing a “product” from a chemical
system.
© 2009 Brooks/Cole - Cengage
79
butane
ButaneIsobutane
Equilibrium
[isobutane]
K=
= 2.5
[butane]
isobutane
© 2009 Brooks/Cole - Cengage
Butane Isobutane
• At equilibrium with [iso] = 1.25 M and
[butane] = 0.50 M. K = 2.5.
• Add 1.50 M butane.
• When the system comes to equilibrium
again, what are [iso] and [butane]?
© 2009 Brooks/Cole - Cengage
80
81
Butane Isobutane
Assume you are at equilibrium with [iso] = 1.25 M and [butane] =
0.50 M. Now add 1.50 M butane. When the system comes to
equilibrium again, what are [iso] and [butane]? K = 2.5
Solution
Calculate Q immediately after adding more
butane and compare with K.
[isobutane]
1.25
Q=
=
= 0.625
[butane]
0.50 + 1.50
Q is LESS THAN K. Therefore, the
reaction will shift to the ____________.
© 2009 Brooks/Cole - Cengage
82
Butane Isobutane
You are at equilibrium with [iso] = 1.25 M and [butane]
= 0.50 M. Now add 1.50 M butane.
Solution
Q is less than K, so equilibrium shifts right —
away from butane and toward isobutane.
Set up ICE table
[butane]
[isobutane]
0.50 + 1.50
1.25
Initial
Change
-x
+x
Equilibrium
1.25 + x
2.00 - x
© 2009 Brooks/Cole - Cengage
83
Butane Isobutane
You are at equilibrium with [iso] = 1.25 M and [butane]
= 0.50 M. Now add 1.50 M butane.
Solution
[isobutane]
1.25 + x
K = 2.50 =
=
[butane]
2.00 - x
x = 1.07 M
At the new equilibrium position,
[butane] = 0.93 M and [isobutane] = 2.32 M.
Equilibrium has shifted toward isobutane.
© 2009 Brooks/Cole - Cengage
Le Chatelier’s Principle
• Change T
– change in K
– therefore change in P or concentrations at
equilibrium
• Use a catalyst: reaction goes to
equilibrium more quickly. K not
changed.
• Add or take away reactant or product:
– K does not change
– Reaction adjusts to new equilibrium “position”
© 2009 Brooks/Cole - Cengage
84
85
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
Kc =
[NO2 ]2
[N2O4 ]
= 0.0059 at 298 K
Increase P in the system
by reducing the volume
(at constant T).
© 2009 Brooks/Cole - Cengage
86
Nitrogen Dioxide Equilibrium
N2O4(g) 2 NO2(g)
Kc =
[NO2 ]2
[N2O4 ]
= 0.0059 at 298 K
Increase P in the system by reducing the volume.
In gaseous system the equilibrium will shift to the side
with fewer molecules (in order to reduce the P).
Therefore, reaction shifts LEFT and P of NO2 decreases
and P of N2O4 increases.
© 2009 Brooks/Cole - Cengage
87
© 2009 Brooks/Cole - Cengage
The Meaning of K
K comes from thermodynamics.
(See Chapter 17.7 – 17.9)
∆G˚ < 0: reaction is product favored
∆G˚ > 0: reaction is reactant-favored
o
Δ
ĘG
= -RT ln K
If K > 1, then ∆G˚ is negative
If K < 1, then ∆G˚ is positive
© 2009 Brooks/Cole - Cengage
88
Thermodynamics and Keq
(Ch. 17.7 – 17.9)
FACT: ∆rGo is the change in free energy
when pure reactants convert COMPLETELY
to pure products.
FACT: Product-favored systems have
Keq > 1.
Therefore, both ∆rG˚ and Keq are related to
reaction favorability.
© 2009 Brooks/Cole - Cengage
89
Thermodynamics and Keq
• The equilibrium point occurs at
the lowest value of free energy
available to the reaction system.
ΔG = 0 = ΔG° + RT ln(K)
ΔG° = –RT ln(K)
© 2009 Brooks/Cole
- Cengage
Copyright
© Cengage Learning.
All rights reserved
90
90
91
Change in Free Energy to
Reach Equilibrium
© 2009 Brooks/Cole
- Cengage
Copyright
© Cengage Learning.
All rights reserved
91
Thermodynamics and Keq
Keq is related to reaction favorability and so
to ∆rGo.
The larger the value of K the more negative
the value of ∆rGo
∆r
o
G
= - RT ln(K)
where R = 8.31 J/K•mol
© 2009 Brooks/Cole - Cengage
92
Thermodynamics and Keq
∆rGo = - RT ln(K)
Calculate K for the reaction
N2O4 2 NO2
∆rGo = +4.8 kJ
∆rGo = +4800 J = - (8.31 J/K)(298 K) ln K
4800 J
ln K = = -1.94
(8.31 J/K)(298K)
K = 0.14
When ∆rGo > 0, then K < 1
© 2009 Brooks/Cole - Cengage
93
ΔG and Keq Practice
Determine the value of ΔrG° and Keq for the
reaction at 25.0°C given the ΔfG° for the
reactants and product. Is reaction product
or reactant favored?
C(s) + CO2(g) 2CO(g)
ΔfG°(kJ/mol) 0
-394
-137
ΔrG° = Σ ΔfG°(product) - Σ ΔfG°(reactants)
ΔrG° = 2(-137) – (-394) = 120 kJ
120,000 J = -8.314 J/(mol*K)*298 ln K
Ln K = 120,000/(-8.314*298) = -48.4
K = 9.23 x 10-22 not spont; reactant fav’d
© 2009 Brooks/Cole - Cengage
94
AP Exam Practice
• 2010B AP Exam #1
• 2008 AP Exam #1
• 2007B AP Exam #1
• 2004B AP Exam #1
• 2003B AP Exam #1
© 2009 Brooks/Cole - Cengage
95









