Variable charge metals
advertisement

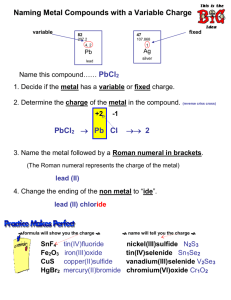
Variable charge metals Ionic Compounds Variable Charge Ions • Some elements in the transition metals can form more than one type of ion. Sometimes they may lose 2, 3, 4 or even more electrons in order to bond with another element. • So these metals can form +2, +3 ,+4 etc cations in one compound but have a different charge in another compound. In order to tell them apart we name them differently. Writing the formula • When given the name of the compound, the Roman numeral after the metal tells us the CHARGE of the metal. • Copper II Chloride – means Cu+2 Cl-1 • Follow the same steps as before >>>> 1. Write out the charges 2. Criss-cross 3. Combine as a formula unit (get rid of charges on top) 4. Simplify if needed Multiple Oxidation States “tin fluoride” Tin is either 2+ or 4+ oxidation state. tin (II) fluoride tin (IV) fluoride Sn2+ Sn4+ F1- SnF2 tin (II) sulfide Sn2+ S2- Sn2S2 SnS F1- SnF4 B. To find the formula, given the name: 1. Write symbols for the two types of ions. 2. Balance charges to write formula. Co Sn cobalt (III) chloride Co3+ Cl1– CoCl3 tin (IV) oxide Sn4+ O2– SnO2 tin (II) oxide Sn2+ O2– SnO Lets Practice some – 1. 2. 3. 4. 5. 6. 7. Lead (IV) oxide Mercury (II) Chloride Bismuth (III) sulfide Iron (II) Bromide Copper (II) Chloride Titanium (III) phosphide Lead (II) oxide When naming…. You need to work backwards from the formula to find the charge of the metal. 1. First write the charge (oxidation state) of the nonmetal (Anion) 2. Multiply the charge by its subscript. 3. The charge (oxidation state) of your cation (metal) multiplied by its subscript needs to equal this number. 4. Write the name of the compound as usual, then change the charge of the metal into a roman numeral, put into parentheses and put it after the name of the metal. A. To name, given the formula: 1. Figure out charge on cation. 2. Write name of cation. 3. Write Roman numerals in ( ) to show cation’s charge. 4. Write name of anion. Fe Cu Stock System of nomenclature FeO iron Fe?2+ oxide O2– Fe2O3 ?3+ oxide iron Fe Fe?3+ O2– O2– O2– iron (III) oxide CuBr ? 1– copper Brbromide Cu1+ copper (I) bromide CuBr2 1– Br1– copper Cu?2+ Brbromide copper (II) bromide iron (II) oxide Let’s practice • • • • • 1. FeCl3 2. CuO 3. Cr2O3 4. NiS 5. V2O5