HONORS CHEMISTRY
advertisement

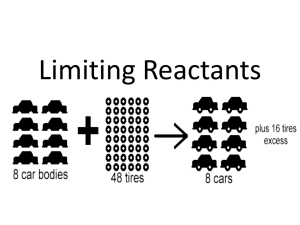
HONORS CHEMISTRY Feb 27, 2012 Brain Teaser Cu + 2 AgNO3 2 Ag + Cu(NO3)2 – How many moles of silver are produced when 25 grams of silver nitrate reacts with excess amount of copper? – How many grams of silver nitrate are needed to produce 50 grams of silver? Agenda • • • • Brain Teaser Notes: Limiting Reactants Work on Mixed Stoichiometry Practice Worksheet 2 Homework – Complete Worksheet Limiting Reactants Limiting Reactants • Reactant that is completely used up in a chemical reaction • Determines the maximum amount of product that can be formed. Limiting Reactants • Why is this important? – The quantities of products formed in a reaction are always determined by the quantity of the limiting reactant. Limiting Reactants • Analogy: making cheese sandwiches Limiting Reactants Limiting Reactants Limiting Reactants 2H2 + O2 2H2O What is the limiting reactant? Limiting Reactants 2H2 + O2 2H2O What is the limiting reactant? Limiting Reactants • How many grams of water can I make from 22 grams of hydrogen and 195 grams of oxygen? – Write a balanced chemical equation H2 + O2 H2O – What is the limiting reactant? • Answer: Do two stoichiometry calculations – Determine mass of product produced by each reactant – How do you know? Determining the limiting reactants • The limiting reagent may not be obvious!! Practice Problem • If 11.3 grams of sodium chloride are formed in the reaction described above, what is the percent yield of this reaction? Can you really get out everything that goes in? % yield = experimental yield x 100 theoretical yield Experimental or actual yield determined in the lab Theoretical or expected yield determined by stoichiometry Practice Problem • If 35 grams of C6H10 reacts with 45 grams of oxygen, how many grams of carbon dioxide will be formed? – What is the limiting reactant? – How much of the excess reactant is left over after the reaction ? – If 35 grams of carbon dioxide are actually formed from the reaction, what is the percent yield of this reaction? Partner Quiz 1) CaCl2 + Na2CO3 CaCO3 + 2NaCl 5.45 g 4.55 g ? How much CaCO3 (in g) is formed? What is the percent yield for this reaction if 3.96 g of calcium carbonate was isolated from the reaction?