Chemical Bonding Notes
advertisement

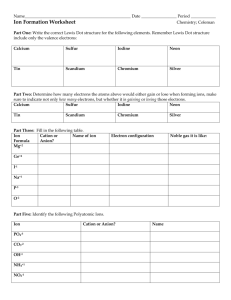
http://player.discoveryeducation.com/index.cfm?guidAssetId=2ED4D53B-8E79453D-B7EE-4DF53F92A9C4&blnFromSearch=1&productcode=US The valence electrons in an atom have HIGH POTENTIAL ENERGY By bonding with other atoms, potential energy is decreased creating stable compounds. Symbols of atoms with dots to represent the valence-shell electrons. (The valence- are the e- directly involved in bonding). Ar Mg C The Noble Gases do not react with other elements because they are already STABLE. The outer most “s” and “p” orbitals are completely filled with 8 electrons, satisfying the octet rule. Ne Elements combine with each other to achieve noble gas electron configurations or (8 outer e-) **The exceptions to the octet rule are Hydrogen and Helium (they only need 2) • The forming of chemical compounds results in chemical change. • Compounds are made up of chemically united elements Compounds have completely different characteristics than its component elements • • Compounds are NEUTRAL 2H2O = H2O = HHO H2O HHO Subscript = # of atoms Coefficient = # of molecules N: 1 H: 5 O: 1 The Gaining and Losing of Electrons to form IONS. (An ion is an atom with a charge.) sharing of electrons • • • • Soluble in water Do not transmit electric current Brittle High melting and boiling points Ionization Energy: the amount of energy needed to remove an e- from an atom. Metals have a low ionization energy Nonmetals have high ionization energy because they are closer to satisfying the octet rule! the higher the electronegativity the greater the ability to pull electron to itself The nature of a bond is determined by differences in electronegativity. The GREATER the electronegativity difference, the MORE ionic the bonding. Ionic compounds result when metals react with nonmetals Metals lose electrons to achieve a stable outer energy level • Positive ions form when electrons are given away • Group 1 metals ion 1+ Group 2 metals ion 2+ Group 13 metals ion 3+ 1. Write the symbol for the cation (+) followed by the symbol for the anion (-). 2. Put any polyatomic ions (see green boxes) in parenthesis 3. Write the oxidation numbers above the appropriate ions 4. Use the criss-cross method to deterimine # of atoms (do not criss-cross ones) 5. Make sure the sum of all ox# is ZERO. 6. Reduce Sodium Atom Sodium Ion Na 11 p+ 11 e0 – e Na + 11 p+ 10 e1+ Fluoride Atom Fluoride Ion + e 17 p+ 17 e0 1- 17 p+ 18e1- Na + 1+ Na 1- YOUR TURN… Using dot diagrams, draw the Lewis structures that result when the following elements form ionic bonds: A. Potassium + chlorine B. Magnesium + fluorine Potassium + Chlorine K Cl + K Cl Magnesium + Fluorine Mg + F Mg F Oxidation: the process of losing electrons ▪ Creates an ion with a + charge ▪ Creates cations Reduction: the process of gaining electrons ▪ Creates an ion with a – charge ▪ Creates anions 1. Write the name of the cation (+) 2. Write the name of the anion (-) with an ide suffix NaCl Sodium and Chlorine Sodium Chloride The oxidation number is not always the same, it varies. The variable oxidation states are represented with a roman numeral in parentheses and are located in groups 3-12 on the periodic chart. Copper Copper (I) = Cu+ Copper (II) = Cu2+ Write the symbol for the cation followed by the symbol for the anion. Write the oxidation numbers above the appropriate ions. Criss-Cross 3+ 2- Copper(III) Oxide: CuO Cu2O3 1+ 2- Copper(I) Oxide: CuO Cu2O SO4 C2H302 NO3 NO2 NH4 HCO3 CO3 OH ClO3 PO4 Sulfate Acetate Nitrate Nitrite Ammonium Bicarbonate Carbonate Hydroxide Chlorate Phosphate 21111+ 12113- 1. Write the name of the cation (+) 2. Write the name of the anion (-) 3. If either is a polyatomic ion write the name of the polyatomic ion (you don’t need to change any suffixes) Na(OH) Sodium Hydroxide Write the symbol for the cation followed by the symbol for the anion. Put all polyatomic ions in parentheses!! Write the oxidation numbers above the appropriate ions. Criss-Cross 2+ 3- 1+ 3- Calcium Phosphate: Ca(PO4) Ammonium Phosphate: (NH4)(PO4) Ca3(PO4)2 (NH4)3(PO4) 1+ 1- =0 Cu(OH) 3+ 62- Ni2O3 Copper (I) Hydroxide =0 Nickel (III) Oxide sharing of electrons Between nonmetallic elements of similar electronegativity. Formed by sharing electron pairs Stable non-ionizing particles, they are not conductors at any state Examples; O2, CO2, C2H6, H2O, SiC Water is a polar molecule because oxygen is more electronegative than hydrogen, and therefore electrons are pulled closer to oxygen. A polar molecule has a (+) and a (-) end. A nonpolar molecule does not have charged ends. Metal + Nonmetal --> ionic compound (usually) Metal + Polyatomic ion —> ionic compound (usually) Nonmetal + Nonmetal —> covalent compound (usually) Hydrogen + Nonmetal —> covalent compound (usually) The chemical symbol for the atom is surrounded by a number of dots corresponding to the number of valence electrons 1. Write the name of the cation (+) with appropriate prefix (do NOT use mono for the cation) 2. Write the name of the anion (-) with the appropriate prefix CO2 Carbon Dioxide 1: 2: 3: 4: 5: 6: mono di tri tetra penta hexa Formula weight is the sum of the atomic masses. Example- CO2 Mass, C + O + O 12.011 + 15.994 + 15.994 43.999