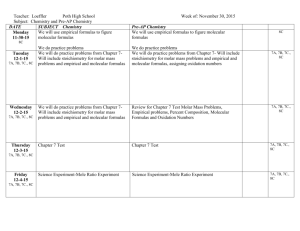
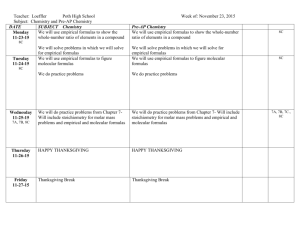
Chapter 25 - Chemical Formulas and Equations
advertisement

Chapter 25 - Chemical Formulas and Equations Section A – Types of Chemical Formula Structural Formula Molecular Formula Empirical Formula Section B – Calculating Empirical Formulas Method 1 – If given the % composition by mass of each element present in the compound 1. Divide the percenntage mass of each element by its _______________________ 2. Find the simplest ___________________________________ between the elements present. Example 1 A compound contains 40% sulfur and 60 % oxygen by mass. What is its empirical formula? Element % moles sulfur 40 40/ oxygen 60 60/ Ratio = 1.25 Ans: Therefore the empirical Formula is St.Dominic’s College Chemistry notes Page 1 Chapter 25 - Chemical Formulas and Equations Method 2 – Given the masses of reactants and products Key idea: Total mass of reactants = 1. Use the masses of reactants and products given to figure out the mass of remaining reactants. 2. Divide the percenntage mass of each element by its _______________________ 3. Find the simplest ___________________________________ between the elements present. Example 2 When 3.175g of copper reacts with chlorine gas 6.725g of copper chloride is formed. Find the empirical formula of the copper chloride Answer: Copper + Chlorine gas 3.175g Element mass Copper 3.175g Chlorine 3.55g Copper Chloride ? 6.725g moles Ratio = 0.05 Therefore the empirical formula is Section C – Molecular Formulas The molecular formula is a multiple of the empirical formula: Molecular Formula = x ( Empirical Formula) x is a ______________ number *****To find the molecular formula we must know the ___________________ and the relative molecular mass of the substance. St.Dominic’s College Chemistry notes Page 2 Chapter 25 - Chemical Formulas and Equations Example 1 Urea is used as a fertiliser and an animal feed. It has a relative molecular mass of 60 and is composed of 46.66% N, 26.66%O, 20%C and 6.66% H. Determine the molecular formula of urea Element % moles ratio nitrogen 46.66 3.33 2 oxygen 26.66 1.66 1 Carbon 20 1.66 1 Hydrogen 6.66 6.66 4 Mass according to Empirical Formula is Relative molecular mass of urea = Empirical formula = x ( Empirical Formula) X= Molecular formula is Section D – Percentage composition by mass The following formula can be used for this: % composition of mass = ______________________________________ x 100 Example 1 What is the percentage by mass of Fe in Fe2O3? Mass of Fe in compound x 100 Relative molecular mass of the Fe2O3 ___________________________ St.Dominic’s College Chemistry notes Page 3 Chapter 25 - Chemical Formulas and Equations Section E – Balancing chemical equations For an equation to be balanced: total number of atoms of each element of reactant must equal the total number of atoms of that element in the product Rules: 1. Chemical formulas can’t be ________________ 2. Chemical formulas can be ________________by a suitable number Example 1 Balance the following equation Step 1: See what is present: Fe + O2 Fe3O4 Reactants Products Oxygen atoms: Iron atoms: Step 2 Make changes to balance the equation: Section F – Balancing redox equations Assigning oxidation numbers can help to balance redox equations. In a redox reaction the number of electrons ____________ from one substance during a reaction must ___________ the amount of electrons __________ by another substance. How to balance a redox equation 1. 2. 3. 4. Assign oxidation numbers to all the species involved in the reaction. Identify what is oxidised (number increases), and how much by. Identify what is reduced (number decreases), and how much by. Identify the ratio needed between what is oxidised and what is reduced so that the total increase in oxidation number is the same as the total decrease in oxidation number. 5. Balance the rest of the equation maintaining the ratio decided on in 4. St.Dominic’s College Chemistry notes Page 4 Chapter 25 - Chemical Formulas and Equations Example 1 MnO4- + Cl- + H+ Mn+2 + Cl2 + H2O Section G – Calculations based on Chemical equations St.Dominic’s College Chemistry notes Page 5 Chapter 25 - Chemical Formulas and Equations Example 1 The fermentation of glucose results in the formation of ethanol and carbon dioxide according to the equation: C6H1206 2C2H5OH + 2CO2 If 126g of glucose are consumed (i) How many moles of glucose does this represent? (ii) How many moles of ethanol are produced? (iii) What volume of carbon dioxide, measured at s.t.p is produced? (iv) How many molecules of carbon dioxide does this volume contain? St.Dominic’s College Chemistry notes Page 6 Chapter 25 - Chemical Formulas and Equations Section H – Reactions involving one reagent in excess Reagent in excess Limiting reagent example 1: Ammonia gas can be prepared from ammonium chloride and sodium hydroxide: NH4Cl + NaOH NH3 + NaCl + H20 If 20.7g of ammonium chloride and 10g of sodium hydroxide are used answer the following questions: Answer (i) which reagent is in excess? (ii) which reagent is the limiting reagent? St.Dominic’s College Chemistry notes Page 7 Chapter 25 - Chemical Formulas and Equations (iii) Calculate the mass of sodium chloride which would be generated in the reaction (iv) the volume of ammonia formed at s.t.p made in the reaction St.Dominic’s College Chemistry notes Page 8 Chapter 25 - Chemical Formulas and Equations Section I – Percentage Yield Percentage Yield = ______________________ Example 1 – In an experiment to prepare ethane 10.2g of ethanol was heated with aluminium oxide and 1.7g of ethene was formed. Calculate the percentage yield of ethene C2H5OH C2H4 + H20 Answer (i) Calculate the theoretical yield: (ii) Calculate the actual yield (iii) Finding the percentage yield: St.Dominic’s College Chemistry notes Page 9 Chapter 25 - Chemical Formulas and Equations Check your learning of chemical equations and formulas.. What you need to know: Green I can do this on my own Orange I can do this with my notes / book but otherwise I get stuck Red I can’t do this yet Definition of empirical formula Calculations of empirical formulas, given the percentage composition by mass. Calculations of empirical formulas, given the mass of reactants and products Definition of molecular formula Calculation of molecular formulas Calculations of the Percentage composition by mass given the empirical formula Definition of Structural formulas and simple examples. How to balance a simple chemical equation How to balance redox equations using oxidation numbers Solve problems involving chemical equations when one reactant is in excess Be able to solve problems involving chemical equations by doing calculations using moles, mass, volumes and numbers of particles. Calculations involving % yields ( see organic chemistry section) St.Dominic’s College Chemistry notes Page 10 Chapter 25 - Chemical Formulas and Equations St.Dominic’s College Chemistry notes Page 11