Basicity of amines

Chapter 19 AMINES
PROBLEMS
2a,b,e,3a,b,c,6a,c16b,c,17a,c,20b,d21b,
25a,c,e,g,26a,c27b,36a,b,37ab,41c,d,I,
42a,b,c44a,c,g
INTRODUCTION
Organic derivatives of ammonia
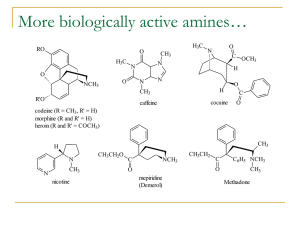
Alkaloids - obtained from plants and have strong biological properties
70% of pharmaceuticals contain nitrogen
Examples
Primary RNH
2
HO
HO
NH
2
CO
2
H CO
2
H
NH
2
HO
HO
TYROSINE
An amino acid that can cross barrier
DOPAMINE
Parkinson's Disease - inadequate concentration of dopamine
Dopamine is a worthless drug since it can't cross blood-brain barrier i.e. can't get in cerebrospinal fluid
Examples continued
Secondary Amines
OH
CH
2
CH
2
CH
3
N
HO
H
CONIINE - Hemlock
SOCRATES CONIINE - Hemlock
HO
SOCRATES
Tertiary Amines
Me
N
O epinephrine
NHMe
O
Me
NHMe methamphetamine speed Tina
Me
N
Me O Me
HO
O
H
Morphine
OH
O
Me OH
H
3
COCO
O
H
HEROIN
OCOCH
3
Nomenclature
Common Names - name radicals attached to nitrogen followed by amine
N sec-butyldiethylamine
NH
2
N-Convention
Me
N
Me
1-aminocyclopentene
N,N-dimethyl-4-methylaniline
Me
IUPAC System
ALKANAMINE - Parent longest chain containing amine
CH
3
CH
2
CH
2
NHCH
2
CH
2
CH
2
CH
2
CH
3
NH
2 N-propyl2-pentanamine
NH
Me
Cl
Cl
3,4-dichloro-N-methybenzenamine
Basicity of amines
The greater the availability of the lone pair electrons on nitrogen, the greater the base.
In the old days, pK b was a measure of base strength.
Kb = [RNH
3
+ ] [OH ] / RNH
2 pK b
= - log K b
The stronger the base the lower the pK b
EFFECTS ON AMINE BASICITY
1. INDUCTIVE EFFECT - ALKYL SUBSTITUTION
CH
3
METHYL GROUP INCREASES ELECTRON DENSITY ON N
CH
3
-NH
2
< CH3-NH
3.36 3.28 2 METHYLS ARE BETTER THAN ONE
WATCH OUT THREE METHYL GROUPS DECREASES
BASICITY pK b
= 4.26 - Steric inhibition of solvation of HOH with the NH + of the R3NH + cation.
The greater the availability of the lone pair electrons on nitrogen, the greater the base.
In the old days, pK b was a measure of base strength.
Kb = [RNH
3
+ ] [OH ] / RNH
2 pK b
= - log K b
The stronger the base the lower the pK b
EFFECTS ON AMINE BASICITY
1. INDUCTIVE EFFECT - ALKYL SUBSTITUTION
CH
3
METHYL GROUP INCREASES ELECTRON DENSITY ON N
CH
3
-NH
2
< CH3-NH
3.36 3.28 2 METHYLS ARE BETTER THAN ONE
WATCH OUT THREE METHYL GROUPS DECREASES
BASICITY pK b
= 4.26 - Steric inhibition of solvation of HOH with the NH + of the R3NH + cation.
2. RESONANCE EFFECT
Base weakening Why?
Delocalizes electron pair on N!!
+
NH
-
NH
2 vs.
NH
2
N H
H
+
N H
9.4
3.
3 aromatic 6 pi system not aromatic pK b
= 15
Hybridization
The greater the % of s character The closer the lone pair is to N The weaker the base
N
H
N
H
3
C N: sp
24 sp
2
8.75
sp
3
2.88
Sections to be omitted
19-7 (salts of amines)
19-8 (amines as PT catalysts)
19-9 (Spectroscopy)
Reaction of Amines to be reviewed
Reaction with Aldehydes and
Ketones - 19-10
Aromatic Substitution of
Arylamines 19-11
Alkylation of Amines
Alkylation of amines by alkyl halides -
Only two situations of importance
Excess ammonia - stops are monoalkylation stage
PhCH
2
Br + excess NH
3
PhCH
2
NH
2
Excess Methyl iodide - all the way to quaternary salt
Propyl-NH
2
+ ex MeI Propyl-N(Me)
3
+
Acylation of Amines
O
R Cl
+
H
H
N
R
O
Ph Cl
+
H
H
N
Me
O
R NHR
AMIDE
O
Ph NHMe
N -methylbenzamide
Mechanism
O
Ph Cl
+
H
H
N
Me
H
+
O
-
Ph NHMe
Cl
O
Ph NHMe
R-NH
2
Amines as Leaving Groups
Hofmann Elimination excess MeI
+
R-NMe
3
I
-
Ag
2
O
+
R-NMe
3
OH
-
H
OH
-
HEAT
-N(Me) 3
-HOH
N(Me)
3
ANTI ELIMINATION - Less stable (less substituted) alkene
4
CH
3
H
H
3
CH
2
Stereochemistry
2
1
CH CH
3
N(Me)
3
+
CH
3
H
Me
CH
3
CH
3 N(Me)
3
+
H looking down C2-C3
H
N(Me)
+ 3
H rotation about C-3 counter clockwise
Suitable for E-2;less stable
More stable; not
Suitable for E-2
Looking down C1-C2
H
+
N(Me)
3
H
H
CH
2
CH
3
H
H
3
CH
2
C
H
H looking down C
1
-C
2
H
H
N(Me)
3
+
Rotating clockwise about C-2 all suitable for E-2 H CH
2
CH
3
H
H
Examples
NH
2 1. excess MeI
2. Ag
2
O heat
Note: NMe3 has no beta hydrogens thus complicating the E-2 process
+
NMe
3 OH
_
H
H
N
Another example
1. excess MeI
2. Ag
2
O h e a t
Me
2
N
+
H
HO
-
NMe
2
Me
2
N
H
NR
2
COPE REACTION
H
2
O
2
_
O
+
NR
2
N-OXIDE
R
2
N
O
-
H
- HO-NR
2
LESS HINDERED BETA HYDROGEN
SYN ELIMINATION
H
3
C
NMe
2
COPE EXAMPLE
H
2
O
2
H
-
O
+ NMe
2
H
2
C
Mild conditions
-HONMe
2
Reaction of Primary Amines with
HNO
2
Preparation of HNO
2
NaNO
2
+
HCl HNO
+
2
NaCl
Reaction
Aliphatic amines
RNH
2
NaNO
2
HCl
RN
2
+ diazonium ion
RN
2
+ R+
-N
2
Hot carbocation
R
+ S
N
1 mixture of products
Aromatic Primary Amines
NaNO
2 ArNH
2
HCl
0 - 5 o C
ArN
2
+ stable undergoes SN1 with nucleophiles
Examples
ArH
H
2
P O
3
ArI
K I
ArOH
ArCN
HO
-
CuCN
ArN
2
+
HBF
4
CuBr ArBr
CuCl
ArCl
ArF
Conversion of NO
2 to NH
Good way to introduce NH
2
2
ArH
HNO
3
H
2
SO
4
ArNO
2
RED
ArNH
2
RED = SnCl
2
/ HCl
R
Nice Synthesis
1. HNO
3
/H
2
SO
4
NH
2
2. SnCl
2
MeCOCl
R
R
R
1. NaNO
2
HCl
2. H
2
PO
3
R
/ RO H
NH
2
Br
HOH
heat
R
Br
NHCOMe
Br
2
/ Fe
NHCOMe
Br
Another one
CH
3
I / AlCl
3
Br
CH
3
Br
Br
2
/ Fe
CH
3
HNO
3
H
2
SO
4
CH
3
Br
NO
2 oxid
CO
2
H
Br
Red
Br
NO
2
NO
2
CO
2
H
Br
1. NaNO3/HCl
2. KI
Br
CO
2
H
Br
NH
2
I
Synthesis of Amines
Reductive Amination
R
N H
1.
R'
C O
2. Reduction
R
N CH
R'
NOTE: carbonyl has been reduced and aminated - most general method
Examples
R
Primary Amines
R
O
NH
2
OH
R
NOH
Red
R OXIME
H
R
R
Could use NH
3 but it is a gas and inconvenient
LAH is usual reducing agent
NH2
R
R
Secondary Amines
R
O
R'NH
2
NR
Red
R from carbonyl compound
R
H
R
Note: Primary amine is converted to secondary amine.
from amine
NHR
R
O
Tertiary Amines
R
R'R"NH
2
+
NR'R"
R
R iminium salt
Very unstabile
So reaction is run with reducing presence at all times.
This means that there will both product and carbonyl compounds presence.
Must use reducing agent that only reduces iminium salt
It is: sodium triacetoxyborohydride!!
Preparation of Tertiary, Con’t
R
+
NR'R"
Na(MeCOO)
3
)
3
BH
R
R
NOTE: Secondary amine converts to tertiary amine
H NR'R"
R tertiary amine
Mechanism of iminium salts
"R
"R
O
-H
2
O
H
NRR'
R"'
+
OH
2
NRR'
>
R"
PROTONATION
"R
"R
R"'
+
..
NRR'
"R
O
-
H
NRR'
+
R"'
OH
NRR'
R"'
+
NRR'
R"' R"'
O
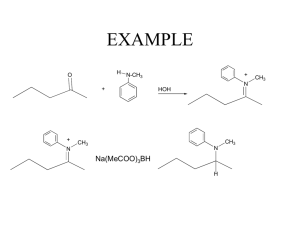
EXAMPLE
+
H
N-CH
3
HOH
+
N
CH
3
Na(MeCOO)
3
BH
N
CH
3
H
+
N
CH
3
Other Ways to Prepare Primary amines
REDUCTION OF NITROANILINES - as before
REDUCTION OF NITRILES
RCH
2
X
CN
-1
RCH
SN2 conditions
2
CN
LAH RCH
2
CH
2
NH essentially replaces halogen with a CH
2
NH
2 group.
Increases carbon chain length by one carbon
Don’t do problem 27b ch.19
Please do problem 30b,
REDUCTION OF AZIDES
RCH
2
X
N
3
-1
RCH
2
SN2 conditions
N
3
LAH RCH
2
NH
2 essentially replaces halogen with an NH
2 group.
No increase in carbon chain length
NOTE 1-bromopentane to 1-pentanamine - azide but 1-bromopentane to1-hexanamide - CN-