ions and ionic bonding
advertisement

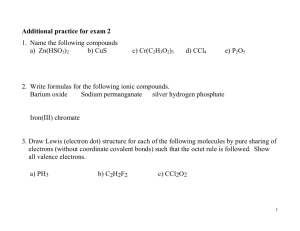
IONS UNIT 1B.10 Ionic Bonding https://www.youtube.com/watch? v=g_hwgHGeRhA https://www.youtube.com/watch ?v=WWc3k2723IM https://www.youtube.com/watch?v =hiyTfhjeF_U Bohr’s Model Electrons move like planets around the sun. In circular orbits at different levels. Amounts of energy separate one level from another. III. The Bohr Model of the Atom A. Electrons of hydrogen circle the nucleus in orbits 1. orbits have a fixed amount of energy in the ground state 2. orbits are a fixed distance from the nucleus 3. orbits furthest from the nucleus have the greatest energy Niels Bohr (1885 – 1962) Bohr Model of the Atom Bohr’s Model Nucleus Electron Orbit Energy Levels Bohr’s Model Fifth Increasing energy Fourth Third Second } First Nucleus Further away from the nucleus means more energy. There is no “in between” energy Energy Levels IONS An atom usually has a neutral charge. That means it has the same number of protons as electrons Remember, a proton has a positive charge and an electron has a negative charge ION – an atom that has lost or gained one or more electrons and has become charged either positively or negatively Positive Ions When an atom LOSES electrons, it becomes more POSITIVE – Why? If you are getting rid of negative particles (electrons) but your number of positive particles (protons) are staying the same. – In other words, you are subtracting negative numbers Examples What would the charge be if: The neutral form of Gold (Au) lost 4 of its 79 electrons. It now has 79 protons and 75 electrons The neutral form of Mg lost 2 of its 12 electrons. It now has 12 protons and 10 electrons. Negative Ions When an atom GAINS electrons it becomes more NEGATIVE – Why? Electrons have a negative charge, so the more you have, the more negative you become Representing Ions Ions are represented by placing a “superscript” charge number next to the atomic symbol. Ex. – O-2 = oxygen with a negative 2 charge – K+ = potassium with a positive 1 charge – N-3 = nitrogen with a negative 3 charge – And so on A. Chemical Bond and valence electron 1.The electrons responsible for the chemical properties of atoms are those in the outer energy level: VALENCE ELECTRONS. a.Valence electrons - The electrons in the outer energy level. b.Inner electrons -those in the energy levels below. Keeping Track of Electrons 2. Atoms in the same column a. Have the same outer electron configuration. b. Have the same valence electrons. c. Easily found by looking up the group number on the periodic table. d. Group 2A - Be, Mg, Ca, etc.2 valence electrons List the number of valence shell electrons are in each of the elements in groups 1-2 13-18 B. Electron Dot Diagrams: Lewis Structures 1. A way of keeping track of valence electrons. 2. How to write them 3. Write the symbol. 4. Put one dot for each valence electron 5. Don’t pair up until they have to X The Electron Dot diagram for Nitrogen Nitrogen has 5 valence electrons. First we write the symbol. Then add 1 electron at a time to each side. Until they are forced to pair up. N Write the electron dot diagram for Na Mg C O F Ne He C. Ion Formation in Representative Elements Group Gain or Lose Charge of Ion 1 (1A) lose 1 +1 2 (2A) lose 2 +2 13 (3A) lose 3 +3 14 (4A) lose or gain 4* +4,-4* 15 (5A) gain 3 -3 16 (6A) gain 2 -2 17 (7A) gain 1 -1 C. Electron Configurations for Cations 1. Metals lose electrons to attain noble gas configuration. 2. They make positive ions. 3. If we look at electron configuration it makes sense. Na 1level- 2e 2 level -8e 3 level -1e: 1 valence electron Electron Dots For Cations Metals will have few valence electrons Ca Electron Dots For Cations Metals will have few valence electrons These will come off Ca Electron Dots For Cations Metals will have few valence electrons These will come off Forming positive ions +2 Ca D. Electron Configurations for Anions 1. Nonmetals gain electrons to attain noble gas configuration. 2. They make negative ions. 3. If we look at electron configuration it makes sense. S 1s22s22p63s23p4: 6 valence electrons S-2 1s22s22p63s23p6: noble gas configuration. Electron Dots For Anions Nonmetals will have many valence .electrons. They will gain electrons to fill outer shell. P -3 P E. Stable Electron Configuration 1.All atoms react to achieve noble gas configuration. 2.Noble gases 8 electrons on last energy level. 3. 8 valence electrons . 4. Also called the octet rule. Ar Write the electron configuration diagram label as anion or cation Na Mg P O F Cl K I. Chemical Bonds A. The force that holds two atoms together. 1. Why do atoms form bonds? a. to acquire 8 electrons in the valence shell (like noble gases 2. How do atoms form bonds? a. atoms may lose, gain or share electrons to get 8 in the valence shell I. Properties of Ionic Compounds Ionic_Bonds.asf View Ionic video a. Crystalline structure. b. A regular repeating arrangement of ions in the solid. c. Structure is rigid. II. Ionic Bonding A. Anions and cations are held together by opposite charges. B. Ionic compounds are called salts. C. Simplest ratio is called the formula unit. D.The bond is formed through the transfer of electrons. E. Electrons are transferred to achieve noble gas configuration. II. Formation and Nature of Ionic Bonds F. 1. atom “M” loses electron(s) cation 2. atom “N” gains electron(s) anion 3. cation and anion attract each other a. electrostatic attraction 4. the electrostatic force that holds the oppositely charged ions together is the ionic bond Sodium loses an electron forming a (+) ion. Chlorine gains an electron forming a ( - ) ion. Electrostatic attraction between the (-) and (+) ion forms the ionic bond between sodium and chlorine B. Ionic Compounds 1. compounds containing ionic bonds 2. types of ionic compounds a. oxides – metal + oxygen Na2O, CaO, Al2O3, Fe2O3 b. salt – metal + nonmetal NaCl CaF AgCl KI c. binary compounds – two elements 1)all of the compounds in a and b are binary compounds D. monoatomic ion - one atom 1) ex. K+ Fe3+ O2- N3E. polyatomic ion – ion with more than one atom that acts as a single ion NO3- OHSO42NH4+ III. Names and Formulas A. Formulas for Ionic Compounds 1. vocabulary a. formula unit – simplest ratio of ions in a compound 1) ex. NaCl MgBr2 AlCl3 b. monoatomic ion - one atom 1) ex. K+ Fe3+ O2- N3- B. Formulas For Ionic Compounds 1. write formula for the cation first, then the anion 2. use subscripts to indicate number of ions (criss-cross the charges) a. sum of charges should equal 0 b. never change subscripts in polyatomic ions Sodium Chloride Crystal Ionic Bonding Na Cl Ionic Bonding: Lewis Structure + Na Cl - Ionic Bonding All the electrons must be accounted for! Ca P Ionic Bonding Ca P Ionic Bonding +2 Ca P Ionic Bonding +2 Ca Ca P Ionic Bonding +2 Ca Ca P -3 Ionic Bonding +2 Ca P Ca P -3 Ionic Bonding +2 Ca P +2 Ca P -3 Ionic Bonding Ca +2 Ca P +2 Ca P -3 Ionic Bonding Ca +2 Ca P +2 Ca P -3 Ionic Bonding +2 Ca +2 Ca +2 Ca P P -3 -3 Ionic Bonding Ionic_Bonds.asf Ca3P2 Formula Unit Shortcut Ca+2 P-3 Ca3P2 Crisscross the charges to become the subscript!!!!!!!!!!!!!!!!!!!!!!!!! I. Properties of Ionic Compounds a. Crystalline structure. b. A regular repeating arrangement of ion in the solid. c. Structure is rigid. Crystalline structure Ionic Compounds NaCl: Ionic compounds consist of a lattice of positive and negative ions. Lattice: three dimensional array of ions Ionic properties d. Ions are strongly bonded- because of strong forces between ions they have 1. High melting points 2. high boiling point 3. high hardness scale 4. very rigid 5. very brittle Ionic solids are brittle + + - + + + + - + + Ionic solids are brittle Strong Repulsion breaks crystal apart. - + - + + - + - + - + E. Conductivity 1.Conducting electricity is allowing charges to move. 2.In a solid, the ions are locked in place. 3. Ionic solids are insulators. 4. When melted, the ions can move around. 5. Melted ionic compounds conduct. 6. First get them to 800ºC. 7. ELECTROLYTE-Dissolved in water they conduct. (aqueous solution) Building Ionic Compounds Binary compound - metallic cation bonded to nonmetallic anion 1. Oxide- When a metal is ionically bonded to Oxygen Salt - Metal + Non-metal Polyatomic Ions An ion made up of two or more atoms bonded together that acts as a single unit with a net charge. Build with tabs NH4 ,OH, Write the polyatomic ion and the charge it has. Na(OH)- a balance compound Mg+2(OH) –1 Mg(0H)2 a balanced compound ACTIVITY: MAKE TAB CUTOUTS OF THE VALENCE SHELL OF AL, O, Na, Cl, Mg, Ca Bond Build just one formula unit- remember it doesn’t exist as one in nature – it is a crystal of many formula units where each – is surrounded by a + Al O Mg O Na Cl Activity Demo- Burn to Create Mg+O=MgO Check the conductivity of: 1. NaCl- solid 2. NaCl-aqueous solution 3. Distilled water 4. Tap 5. MgO-solid 6. MgO-aqueous solution 7. use magnifying glass to view NaCl I. Metallic Bonds How atoms are held together in the solid form. Metals hold onto their valence electrons very weakly. Think of them as positive ions floating in a sea of electrons. http://www.youtube.com/watch?v=c4udBSZf LHY Sea of Electrons Electrons are free to move through the solid. Metals conduct electricity. + + + + + + + + + + + + Metals are Malleable Hammered into shape (bend). Ductile - drawn into wires. Malleable + + + + + + + + + + + + Malleable Electrons allow atoms to slide by. + + + + + + + + + + + + Video http://www.youtube.com/watch?v=Bjf9gMDP 47s ALLOYS Alloys are solid solutions made by dissolving metals in other metals. They are prepared by melting the metals together and cooling the mixture. The properties of alloys differ from those of their component metals. For example stainless steel, an alloy of iron, carbon, chromium and nickel is stronger than iron and more resistant to corrosion. Name of Alloy Composition ________________________________________ Sterling silver silver, copper Brass copper, zinc, tin Cast iron iron, carbon Steel iron, carbon Stainless steel iron, chromium, carbon, nickle 18 Carat gold gold, silver, copper Pewter tin, copper, bismuth, antimony Plumber’s solder lead, tin ALLOY A combination of 2 metals IV. A. Metallic Bonds – Properties of Metals Metallic Bonds 1. valence electrons are delocalized a. free to move from atom to atom 2. bond is formed by the attraction of metal cations for the moving electrons 3. “electron sea model” –atoms of metals contribute a “sea” of free moving electrons that move from one atom to another Electron Sea Model for Metallic Bonds Positive Ions Surrounded by Delocalized Electrons B. Properties of Metals 1. moderately high melting points 2. high boiling points 3. malleable a. can be hammered into sheets 4. ductile a. can be drawn into wire 5. good conductors of heat and electricity 6. luster (good reflectors of light) 7. hardness and strength varies a. greater in transition elements C. Alloys 1. mixture of two or more elements with metallic properties 2. types a. substitutional – atoms of similar size (sterling silver, brass, pewter) b. interstitial – small holes in the crystal filled with smaller atoms (carbon steel)