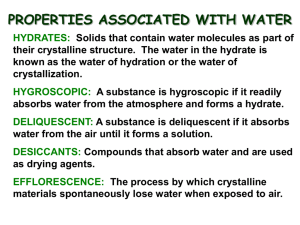
Title: Composition of Hydrates Lab Purpose: The purpose of this
advertisement

Title: Composition of Hydrates Lab Purpose: The purpose of this experiment is to determine the percent of water in a hydrate. Materials: -Evaporating dish -Wire Gauze -Laboratory balance -Copper (II) sulfate hydrate -Iron ring -Microspatula -Safety glasses -Crucible tongs -Bunsen burner -Ring stand Procedure: 1. Prepare the setup as shown. 2. Heat the dish with the hottest part of the flames for 3 minutes. 3. Turn the Bunsen burner off, and allow the dish to cool for 3 minutes. 4. Find the mass of the evaporation dish to ± 0.01 g. Record the mass in the data section. 5. With evaporation dish on the balance, measure into it approximately 2.00g copper (II) sulfate hydrate. Record the mass of the evaporating dish and the hydrate in the data section. 6. Place the evaporating dish with the hydrate one the wire gauze. Gently heat the dish by moving the burner back and forth around the base of the evaporating dish. Avoid any popping and spattering caused by keeping the burner stationary. 7. Heat strongly for 5 minutes or until the blue color has disappeared. During heating, a microspatula may be used to spread the solid and break up any caked proportions of the hydrate. If the edges of the solid appear to be turning brown, remove the heat momentarily and resume heating at a gentler rate. 8. Allow the evaporation dish to cool for about 5 minutes. Immediately, find the mass of the dish with the anhydrous salt and record it in the table. Data Table: Mass of evaporating dish 21.13 g Mass of evaporating dish + hydrate 23.21 g Mass of evaporating dish + anhydrous salt 22.47 g Analysis/Calculations: 1.) The color of the hydrate before any heating was done was blue. 2.) Mass of the hydrate was 2.08 g. Mass of evaporating dish + hydrate Mass of evaporating dish Mass of hydrate 23.21 g - 21.13 g 2.08 g 3.) The color of the anhydrous salt was white/gray. 4.) The mass of the anhydrous salt was 1.34 g. Mass of evaporating dish + anhydrous salt Mass of evaporating dish Mass of anhydrous salt 22.47 g -21.13 g 1.34 g 5.) The mass of the anhydrous salt is different from the mass of the hydrate because during the experiment the water in the hydrate was evaporated, making the weigh less. 6.) The number of moles in the anhydrous salt was .0083 mol of CuSO4. 1.34 g CuSO4 1 mol CuSO4 = .0083 mol CuSO4. 161.45 g CuSO4 7.) The mass of the water lost was 0.74 g. Mass of hydrate 2.08 g - Mass of anhydrous salt -1.34 g Mass of water .74 g 8.) The number of moles of water was .041 mol of H2O .74 g H2O 1 mol H2O = .041 mol H2O 18.02 g H2O 9.) There was 35.6% of water in the hydrate. Mass of water x 100 (0.74 g / 2.08g) x 100 Mass of hydrate = 35.6 % 10.) The value of x in the formula CuSO4*xH2O is 4.94. .041 mol of H2O = 4.94 .0083 mol of CuSO4 11.) The experimental error in this lab is 1.11%. |36.0 -35.6 | x 100% = 1.11% 36.0 Good result, but need to round to a whole number (5), since it’s part of a formula. Good info, but belongs later in the conclusion 12.) The mass of the anhydrous salt must be measured immediately upon cooling to avoid letting any water molecules form and alter the actual mass of the anhydrous salt. CuSO4•H2O Conclusion: The purpose of this experiment was to determine the percent of water in a hydrate of copper (II) sulfate (CuSO4), and it was done successfully with only an experimental error of 1.11%. The mass of the water was found by heating the CuSO4 with a Bunsen burner, and then immediately massing the sample. During this experiment the sample of CuSO4 was massed twice, before heating it and after. As shown above the mass of the CuSO4 hydrate was 2.08 g, and after it was heated it had a mass of 1.34 g. This way the difference was easily found to be 0.74 g. Finally, by using formulas shown above, the percent of the water was calculated which was 35.6%. When the CuSO4 was heated there was also a change of color. The sample went from being blue to gray. The percent error, as stated before, was 1.11%. This is very reasonable due to the materials used, which although were efficient, were very basic. The procedure for the experiment could be improved by doing it multiple times to have better knowledge of the actual percent of water in the hydrate. This experiment relates to what is currently being studied in class because the percent of water could not have been determined without knowing how to find the mole of the different samples, which is what we have been focusing on in class has been the recent focus of class. In conclusion, the goal of the experiment was achieved by using the mole, and doing careful calculations, and because of the minor percent error it was very successful.