School “Flu” Clinics 2012-2013
advertisement

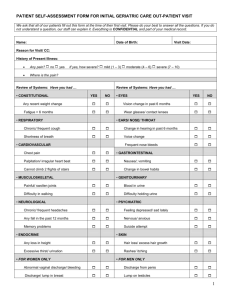
School “Flu” Clinics 2013-2014 Arkansas Department of Health Northwest Region Nursing • Non ADH and ADH Non-Clinical – Attend orientation • Non-ADH nurses – Provide current Arkansas Nursing License – Picture ID – Sign Volunteer Indemnity form – Sign HIPPA form Goals and Objectives • • • • • • • Provide protection against influenza Decrease Illness Decrease Absentees Decrease Spread of Disease Community Immunity Herd Immunity Cocoon Effect Indications for Flu Vaccine • Any person who wishes to reduce the likelihood of becoming ill or transmitting influenza • Inactivated influenza vaccine can be given to anyone 6 months of age or older, including breastfeeding and pregnant women • LAIV vaccine can be given to healthy, nonpregnant persons 2 through 49 years of age Recommendations • All persons aged ≥ 6 months, unless medically contraindicated • Persons at higher risk for influenzarelated complications Recommendations • Adults and children who are immunosuppressed • Adults and children who have any condition that can compromise respiratory function or the handling of respiratory secretions or that can increase the risk for aspiration • Residents of nursing homes/chronic-care facilities When vaccine supplies are limited, vaccination efforts should focus on delivering vaccination to the following persons • Children aged 6 months through 4 years (59 months) • Persons aged ≥ 50 years • Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus) • Persons who have immunosuppression (including immunosuppression caused by medications or by HIV infection) • Women who are or will be pregnant during the influenza season • Children and adolescents (aged 6 months through 18 years) who are receiving long-term aspirin therapy Continue • Residents of nursing homes and other long-term care facilities • American Indians/Alaskan Natives • Persons who are morbidly obese (body-mass index ≥ 40) • Health-care personnel • Household contacts and caregivers of children aged < 5 years and adults aged ≥ 50 years, with particular emphasis on vaccinating contacts of children aged < 6 months • Household contacts and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza To prevent transmission to those who are at high risk for influenza-related complications: Immunize the people who live with or care for them! People Who are Transmission Sources • Healthy household contacts and caregivers of – children ages 0-59 months – persons > 50 years old – persons at high risk for severe complications from influenza Transmission Sources continued Health Care Providers: • People who provide home care to persons in groups at high risk • People working in health-care settings include physicians, nurses, and other workers in hospitals, long term care facilities, assisted living and outpatient-care settings • Medical emergency response workers • Students in these professions who will have contact with patients Influenza Vaccine 2013-2014 • Injectable (IIV3 /Fluzone and RIV3/ Flublok) – A/California/7/2009 (H1N1) -like virus – A/Victoria/361/2011 (H3N2)-virus – B/Massachusetts/2/2012-like virus • Intranasal (LAIV/FluMist and Fluarix Quadrivalent) »Additional B/Brisbane/60/2008-like virus Vaccine • Approx 2 weeks after vaccine-antibodies develop • Common reactions (Injectable) – Soreness/erythema/induration at site lasting 1-2 days (15-20%) – Fever/chills/malaise/myalgias lasting 1-2 days (<1%) – Rare: immediate hypersensitivity allergic reactions (hives/angioedema/allergic asthma/systemic anaphylaxis) Adverse Reactions LAIV • • • • • • • Runny nose or nasal congestion Fever > 100°F Sore throat Headache Vomiting Abdominal pain Myalgias Age Group Dosage IIV3/IIV4:Injectable • 6 through 35 months 0.25 mL IM* – Fluzone (IIV3) • 36 months through 8 years 0.50 mL IM* – Fluzone (IIV3) – Fluarix (IIV4)** • ≥ 9 years 0.50 mL IM – Fluzone (IIV3) • 36 months through 18 years 0.50 mL IM* – Fluarix (IIV4)** • *May need two doses administered at least 4 weeks apart – **Reserved for children with asthma/limited doses available in LHU RIV3: Injectable • 18-49 years of age 0.50ml • Utilized for ADH employees with egg allergies • Available from Central Office LAIV- FluMist® (Live Attenuated Intranasal Vaccine) • Approved for healthy, nonpregnant persons 2 through 49 years of age • Children 2 through 8 years of age may need two doses of LAIV administered at least 4 weeks apart • One dose of LAIV may be administered by the intranasal route to persons 9 through 49 years of age Age Group Dosage LAIV-FluMist® • Age: 2 years through 49 years • Prefilled, single-use sprayer containing 0.2 mL of vaccine – If not simultaneously administered, FluMist can be administered 4 weeks after another live vaccine such as MMR or varicella – Do not administer FluMist until 48 hours after antiviral cessation – Antiviral agents should not be administered until 2 weeks after FluMist administration unless medically necessary Contraindications: All Flu Vaccine (IIV/LAIV) • People who had a severe (anaphylactic) reaction to chicken eggs* • People who had a severe reaction to a flu vaccination in the past • Children <6mo of age • People who developed Guillain-Barre’ syndrome following influenza vaccination* – *requires a consultation with patient’s physician Contraindications and Precautions to the Use of Influenza Vaccines: 2013-14 Vaccine IIV (includes IIV3, and IIV4), Fluzone and Fluarix Contraindications Precautions Moderate to History of severe severe illness with allergic reaction to or without fever. any component of the History of vaccine, including egg Guillain-Barre protein, or after syndrome within 6 previous dose of any weeks of receipt influenza vaccine. of influenza vaccine. Contraindications and Precautions LAIV (Flumist) History of severe allergic reaction to any component of the vaccine, including egg protein, gentamicin, gelatin, and arginine, or after a previous dose of any influenza vaccine Concomitant Aspirin therapy in children and adolescents Children aged 2--4 years whose parents or caregivers report that a health-care provider (HCP) has told them during the preceding 12 months that their child had wheezing or asthma Moderate to severe illness with or without fever. History of Guillain-Barre syndrome within 6 weeks of receipt of influenza vaccine. Contraindications LAIV continued • Persons ages <2 years or those ages >49 years • Persons with any of the underlying medical conditions – – – – Asthma Reactive airways disease Chronic disorders of the pulmonary or cardiovascular systems Metabolic diseases diabetes, renal dysfunction, and hemoglobinopathies – Known or suspected immunodeficiency diseases or immunosuppressed states • Pregnancy • Vaccination within 4weeks with live virus vaccine Influenza vaccine dosing algorithm for children aged 6 months through 8 years Injection Technique “Nursing 101” Vaccine Administration TIV(Injectable) • Sites: – Infants: vastus lateralis (anterolateral thigh) – Young children over the age of 12 months-anterolateral aspect of the thigh may be used if the deltoid is underdeveloped – Adults and older children: deltoid (upper arm) • Dosage: – Infants 6mo through 35mo of age: 0.25ml IM – 3 years old and older : 0.5ml IM (Vaccinator assistants (physicians and nurses) may prefill syringes with vaccine preparing just enough vaccine to meet the clinic’s needs on an ongoing basis. Discard any vaccinefilled syringes after the clinic closes.) Injection Technique “Nursing 101” (#1) • Determine appropriate injection site • (Please note that the ADH will be using Safety syringes and needles) Injection Technique, Continued (#2) • Prep site with alcohol wipe/cotton ball • Using circular motion/wipe from center out/allow to dry Injection Technique, Continued (#3) • Spread skin taut between thumb and forefinger OR grasp tissue and “bunch up” muscle. (acceptable for pediatric and geriatric patients) • Insert needle fully into muscle at 90 degree angle and inject vaccine quickly. • While needle is still in the patient, fully depress the plunger to activate retraction . “Nursing 101” • Apply light pressure to injection site for several seconds with dry cotton ball or gauze • Dispose of syringe in sharps container Administering LAIV Step by Step x5 Note: Active inhalation “sniffling” is not required by the patient during administration Dispose used LAIV applicator in Hazardous Waste Bucket Standard Precautions Hand washing-if soap and water not available-use alcohol-based waterless cleanser between each patient (available at each nursing station) Standard Precautions continued. Gloves-not mandatory unless provider has open lesion on hands. (Available at each nursing station) If latex free gloves required-contact nursing coordinator Needle Safety DO NOT: (If not utilizing safety syringes or needles or needle fails to retract) – Detach used needles from syringe – Recap used needle – Bend or break used needle before disposing If needle stick injury occurs-report IMMEDIATELY to the nursing coordinator ADH Supplies • Nursing staff/clerical staff • Vaccine • Syringes/cottonballs/bandages/biohazard containers/tissue • Hand sanitizer/gloves • Trash bags • Emergency kit • Extra forms/pens/clipboards School provides • • • • • • • Adequate space/site School Nurse(s) Volunteers Tables/chairs (set up prior to clinic) Trash cans Completed paper work/forms Faculty/staff copy of insurance card Forms • Vaccine Information Statements – LAIV (mist) – IIV (injectable) • • • • • Consent to Receive Vaccine Form “Dear Parent” letter Will be collated/stapled/50 per set Spanish forms separate/blue print FERPA* form provided by school – Forms may be available prior to start of school/will be shipped weekly when available Volunteers reviewing forms Communicating between office and clinic site School district provided busing for staff/volunteers between multiple campuses same day clinic Hotwash/debriefing with Local Health Unit and school staff/volunteers Questions and Suggestions