File
advertisement

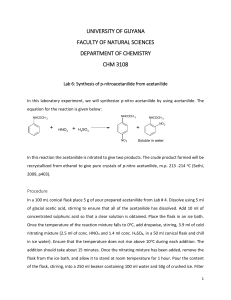
Kaitlin Webb Chemistry 112lab write up Experiment #2 Recrystallization In this particular experiment it involved dissolving an impure compound in a hot solvent and adding the decolorizing agent. Then they had to cool the filtrate until the compound crystallizes. Then they had to wash the crystals that were formed with solvent and dry them to remove any last solvent that could have been left over. The experiment continued with the weighing of 4.00 grams of impure acetanilide. Then was placed in an Erlenmeyer flask. Then they added 70mL of water and 4 boiling chips until it started to boil. After the acetanilide boiled then they had to pour it through filter paper. Crystals began to form and the cooling process continued. They then weighed the crystals that were formed and determined the melting point range. They then had to calculate for the percent recovery that they ended up with which goes as follows. The unknown sample was sample C and the mass of the impure acetanilide was about 3.97 grams. The mass of purified acetanilide was .4163 grams. The melting point range was 109112 degrees Fahrenheit. So they conclude that the percent recovery was grams of purified acetanilide/ grams of impure acetanilide times 100%. This looked like .4163/3.97x100% which equals 11.62%. This concludes that 11.62% of purified acetanilide was in the unknown sample C. 82.4% of the impure material was in our unknown sample. When it was compared to the first experiment that had to do with melting points, we concluded that the substance was closest to urea. So therefore the unknown substance was urea.