pptx
advertisement
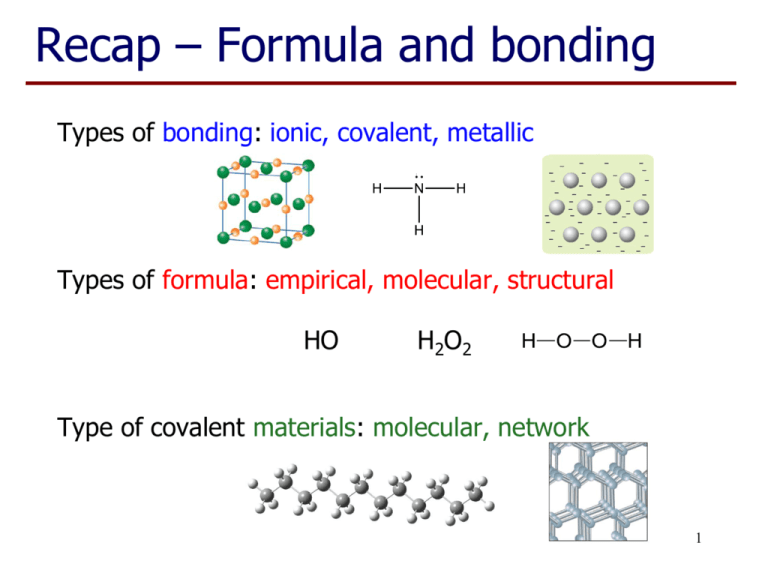
Recap – Formula and bonding Types of bonding: ionic, covalent, metallic Types of formula: empirical, molecular, structural HO H2O2 Type of covalent materials: molecular, network 1 Polarity of Water Fig. 4.2 Silberberg • The O-H bonds in water are polar. • The angular shape of the molecule mean water is a ‘polar molecule’. 2 Chemical Reactions Chemical reactions occur because: • Products contain less energy than reactants and systems go to lowest energy state, eg burning gas. • Energy supplied to force reactants to products which have higher energy, eg blast furnace. 3 Chemical Equations • Word Equation hydrogen plus oxygen forms water • Symbolic Equations – use correct formula H2 + O2 H2O • Need to balance equations. Indicate states 2H2(g) + O2(g) 2H2O(l) 4 Chemical Equations • Molecular Equation eg H2(g) + I2(g) 2HI(g) C6H12O6(s) C6H12O6(aq) • Ionic Equations eg NaCl (s) Na+(aq) + Cl-(aq) Pb2+(aq) + 2I-(aq) PbI2(s) Precipitate 5 Reactions with Acid, H+ • H+ cation is just a ‘bare proton’, no e-. - e- Hydrogen atom H+ Hydrogen ion • In aqueous solution, H+ associates with H2O to give H3O+(aq), also called H+(aq). + H O H H • Substances that provide H+ ions in water are called ACIDS. 6 Metal + Acid Salt + Hydrogen Formula Equation Zn + 2HCl ZnCl2 + H2 Complete ionic equation Zn(s) + 2H+(aq) + 2Cl-(aq) Zn2+(aq) + H2(g) + 2Cl-(aq) Net ionic equation Zn(s) + 2H+(aq) Zn2+(aq) + H2(g) We can isolate the salt by evaporation of the solvent Zn2+(aq) + 2Cl-(aq) ZnCl2(s) 7 Carbonate + Acid Salt + H2O + CO2 Formula Equation CaCO3 + 2HCl CaCl2 + H2O + CO2 Complete ionic equation CaCO3(s) +2H+(aq) + 2Cl-(aq) Ca2+(aq) + H2O(l) + CO2(g) + 2Cl-(aq) Net ionic equation CaCO3(s) + 2H+(aq) Ca2+(aq) + H2O(l) + CO2(g) We can isolate the salt by evaporation of the solvent 8 Ca2+(aq) + 2Cl-(aq) CaCl2(s) Learning Outcomes: • By the end of this lecture, you should: − − − − − − − − understand the reason that water dissolves some ionic materials know the component parts of a chemical equation be able to balance a chemical equation recognise a molecular equation, formula equation and an ionic equation be able to describe a chemical reaction in terms of a chemical equation understand an acid supplies H+ ions and exists in water recognise reactions involving dissolution, precipitation and acids be able to complete the worksheet (if you haven’t already done so…) 9 Questions to complete for next lecture: 1. Balance the following chemical equations: a) CH4(g) + O2(g) CO2(g) + H2O(l) b) CaCl2 c) Ag+(aq) + CrO42-(aq) Ag2CrO4(s) d) Ca(s) + H+(aq) Ca2+(aq) + H2(g) e) Mg(OH)2(s) + H+(aq) Mg2+(aq) + H2O(l) + AgNO3 Ca(NO3)2 + AgCl 2. Classify the above equations as ‘molecular’, ‘formula’, or ‘net ionic’. 3. Which of the equations in question 1 represent a precipitation reaction? 4. Which of the equations in question 1 represent a reaction with acid? 5. Would it matter if you used hydrochloric acid or sulfuric acid to perform the reaction represented by equation 1d? 10





