Chemistry AS Scheme of Work 2011-2012 Unit F321: Atoms, Bonds
advertisement

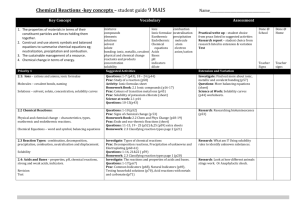
Chemistry AS Scheme of Work 2011-2012 Unit F321: Atoms, Bonds and Groups Lesson Num 1 Lesson Title Learning Objectives Atomic Structure 1) Describe the structure and properties of the atom Module 1.1 2) Explain the distribution of mass and charge in an atom 3) Explain the contribution of protons and neutrons to the atomic and mass number. Prac 2 Ions and isotopes 1) Be able to deduce the atomic and mass number. 2) Be able to deduce the ion from its Starter Main Plenary atomic structure 3) Understand the term isotope and be able to deduce number of protons and neutrons. Prac 3 Masses 1) Understand the term relative isotopic mass, molecular mass, and relative formula mass 2) Understand the relevance of 12C as a standard measurement of mass 3) Be able to calculate the relative atomic mass given relative abundances of isotopes. Prac 4+5 The mole 1) Understand the terms “amount of substance” and “mole” and “Avogadro’s constant NA” 2) Define and use the term ‘molar mass’ with units g mol-1 3) Know the terms empirical formula, molecular formula and be able to calculate them using composition by mass and percentage compositions. Prac Formation of Potassium Iodide demo Calculate formula from initial masses. Atomic mass of metal based on collection of hydrogen from reaction with acid. 6+7 Reacting masses 1) Know the value of 1 mole for mass, gas volume and liquid volumes and concentrations 2) Be able to establish balanced compounds from mol calculations 3) Evaluate the terms concentrated and dilute for solutions Prac 8+9 Produce different concentration solutions using volumetric flask and distilled water Acids and Bases 1) Explain that acids release H+ ions in aqueous soln, and know the formula for common acids. 2) Know that metal oxides, metal hydroxides and ammonia are common bases 3) State that an alkali is a soluble base, releases OH- ions in soln, and know the formula of common alkalis Prac 10 pH probe to demonstrate a precise measure of pH. Record as alkali is added to acid. Salts 1) Describe the reactions of an acid with carbonates, bases and alkalis to form a salt 2) Explain that salts are formed, when the H+ of an acid is replaced by the metal or NH4+ Prac 3) Explain that a base readily accepts H+ ions (example include OH- and NH3) Produce and ammionium salt from ammonia and acid reaction 11-12 Salts (II) 1) Explain the terms anhydrous, hydrated and water of crystallization 2) Be able to calculate the formula of a hydrated salt from percentage composition, mass composition or experimental data. 3) Carry out acid/base and structured titrations. Anhydrous copper sulfate – record mass. Add water slowly – record mass of hydrated copper sulfate. Calculate the formula: CuSO4.5H20 Prac Titration – establish RFM of washing soda by titration. 13-14 Oxidation number 1) Understand the term oxidation number 2) Be able to explain oxidation and reduction in terms of electron transfer and change in oxidation number 3) Be able to apply the roman numeral to indicate oxidation number for a variety of ions and compounds. TBA Prac 15 Redox Reactions 1) Explain why metals generally lose electrons and non-metals gain electrons – and what does this mean for the oxidation number. 2) Describe redox reactions with HCl and H2SO4 3) Make predictions about oxidation numbers based on equations. TBC Prac 16 Module 1.2 Ionisation energies 1) Describe the term first ionisation energy, and second ionisation energy. 2) Explain ionisation energies in terms of nuclear charge, electron shielding and size of atom. 3) Predict an element from first and successive ionisation energies. Prac 18 - 19 Electron configuration 1) Name and order the 4 electron shells (and sub shells), and know how the orbitals are filled up to element 36. 2) Be able to describe and draw s and p orbitals. 3) Describe the relative energies for the shells and relate this to s- pand d- block elements. Prac 20 Ionic bonding 1) Describe ionic bonding as electrostatic attraction between oppositely charged ions. 2) Construct ‘dot and cross’ diagrams for a variety of ionic compounds. 3) Make predictions about ionic charge of ions based on periodic table (including polyatomic ions e.g. SO42-). Prac 21 Covalent bonding 1) Describe covalent bonding, with examples of single and multiple bonds. 2) Explain how dative covalent bonding (coordinate) differs from regular covalent bonding 3) Be able to draw dot and cross diagrams of Cl2, HCl, H2O, NH3, CH4, BF3 and NH4+, SF6 Prac 22 Shapes of molecules and ions 1) Explain the shape of simple molecules based on repulsion of electron pairs around a central atom. 2) Understand that lone pairs repel more than bonded pairs 3) Explain the shapes and bond angles in trigonal planar, tetrahedral, octahedral, pyramidal, non-linear and linear. Balloons modelling Prac 23 Electronegativi ty and bond polarity 1) Describe the term electronegativity as the ability of an atom to attract bonding electrons in a covalent bond 2) Explain how a permanent dipole may form when covalently bonded atoms have different electronegativity Prac 24 Intermolecular forces 1) Describe intermolecular forces based on permanent dipoles, induced dipoles (van der Waals forces), and noble gases. 2) Describe hydrogen bonding, including the role of the lone pair, in water, ammonia and –OH and – NH groups. 3) Explain the link between density and the melting and boiling points of water – and hydrogen bonding. Surface tension experiment(?) Prac 25-26 Bonding and physical properties 1) Describe metallic bonding as the attraction of positive ions to delocalized electrons. 2) Describe the following structures: giant ionic lattice, giant covalent lattices, giant metallic lattices and simple molecular lattices (eg ice) 3) Interpret physical properties from different bonding types above: Melting Points/Boiling Points Electrical conductivity Solubility Prac 27 Module 1.3 Periodic Table Groups and Periods 1) Describe the order of the elements in terms of increasing atomic number – with periods and groups. 2) Describe periodicity in terms of repeating patterns across different periods. 3) Explain why elements in the same group have similar chemical and physical properties – with discussion of electron configuration Prac 28 Periodic Table Physical 1) Explain the changes in ionisation energy of elements across each properties period, and down each group (refer to nuclear charge, atomic radius and electron shielding). 2) Describe and explain the variations in melting and boiling points across periods 2 and 3 in terms of structure and bonding. 3) Be able to interpret data on electron configuration, atomic radii, first ionisation energies, m.p. and b.p. to describe periodicity Prac 29 - 30 Group 2 Redox reactions 1) Describe the redox reactions of the Group 2 elements – with oxygen and water – and the use of Ca(OH)2 in agriculture and Mg(OH)2 in indigestion tablets. 2) Explain the trend in reactivity of Group 2 elements down the group due to increasing ease of cation formation (atomic size, shielding, nuclear attraction) 3) Explain the reactions of Group 2 oxides and carbonates – including trends in thermal decomposition of carbonates and resulting solutions from Gp 2 oxides with water. 4) Interpret and make predictionsfrom chemical and physical properties of Gp 2 compounds. Prac Decomposition of Group 2 carbonates – observe speed of reaction (colour change?) Reaction of Gp2 oxides with water – analysis of pH. 31 Group 7 Properties Titration of indigestion tablets – pH probe precision results and graph. 1) Explain the trend in boiling points of Cl2, BR2, and I2 in terms of Van der Waals’ forces. 2) Explain reactivity of the halides with reference to negative ions, atomic size, shielding and nuclear attraction. 3) Interpret and make predictions from the chemical and physical properties of Gp 7 elements and their compounds. Prac 32 Group 7 Redox reactions 1) Describe the redox reactions of Gp 7 elements, including ionic reactions, in the presence of organic solvent to illustrate relative reactivity of Gp 7. 2) Describe the term disproportionation as a reaction in which an element is simultaneously oxidized and reduced. 3) Contrast the benefits and associated risks of using chlorine in water treatments. Prac Halogen displacement reactions 32 Group 7 – Characteristic reactions 1) Describe the precipitation reactions, including ionic equations, of the aqueous Cl-, Br- and I- with silver ions followed by aqueous ammonia. 2) Describe the use of precipitation reactions as a test for the different halide ions. Prac Halogen displacement reactions Unit F322 – Chains, Energy and Resources Lesson Num Lesson Title Learning Objectives 1 Representing organic compounds 1) Be able to recognise the following representations “empirical formula”, “molecular formula”, general formula, Module Starter Main Plenary 2.1 structural formula, displayed formula, “skeletal formula” correctly 2) Be able to use the above terms correctly 3) Be able to convert one representation into another Prac 2 Homologous series 1) Be able to state the name of the first ten members of the alkane series 2) Be able to use the term homologous series and functional group, with examples 3) Use a general formula of a homologous series to predict the formula of any member of the series. Prac 3 IUPAC rules 1) Be able to recognise and and name common organic functional groups 2) Be able to use the systematic naming method to name simple compounds featuring common functional groups 3) Be able to use the systematic naming method to name more complex compounds featuring common functional groups Prac 4 Isomerism 1) Be able to describe and explain structural isomers, stereoisomers, E/Z isomerism and cis-trans isomerisation. 2) Be able to explain the difference between cis/trans and E/Z stereoisomerism. 3) Be able to determine possible structural formulae and stereoisomers of an organic compounds. Prac 5+6 Reaction Mechanisms 1) Describe the different types of covalent bond fission (homolytic and heterolytic) 2) Describe a curly arrow as the movement of an electron pair, showing breaking or formation of a covalent bond. 3) Be able to draw reaction mechanisms to show the movement of an electron pair with curly arrows. Prac 7+8 Percentage yields and atom economy 1) Be able to perform percentage yield calculations. 2) Be able to explain atom economy, and perform associated calculations 3) Explain that addition reactions have an atom economy of 100%, but substitution reactions are less efficient. 4) Describe the benefits of developing chemical processes with a high atom economy. 5) Explain that a reaction may have a high percentage yield but a low atom economy. Prac 9 Hydrocarbons from crude oil 1) Explain the use of crude oil as a source of hydrocarbons separated by fractional distillation and used as fuels of processing into petrochemicals. 2) State that alkanes and cycloalkanes are saturated hydrocarbons, and explain the tetrahedral shape around each carbon atoms in alkanes. 3) Explain the variations in boiling points of alkanes with different chain lengths and branching in terms of van der Waals forces. Prac Fractional distillation and investigation of properties of fractions. 10 Hydrocarbons as fuels 1) describe combustion of alkanes, leading to use of fuels in industry, homes and transport. 2) Explain, with the use of equations, the difference between complete and incomplete combustion and the dangers arising from the problems of CO. 3) Describe the use of catalytic cracking to obtain more useful alkanes and alkenes. Observe differences between the different combustion types when using a Bunsen burner / burning splints. Prac 11 Hydrocarbons as fuels (II) 1) Explain that the petroleum industry processes straight chain hydrocarbons into branched alkanes and cycloalkanes to promote efficient combustion. 2) Be able to contrast the value of fossil fuels for providing energy and raw materials with an over reliance on non renewable fossil fuel reserves and the importance of developing renewable plant based fuels. 3) Be able to contrast the value of fossil fuels for providing energy and raw materials with increased CO2 levels from combustion of fossil fuels leading to climate change and global warming. Energy of combustion investigation, compare straight chain and cycloalkane combustion. Prac 12 Substitution reactions of alkanes 1) Define the term radical as a species with an unpaired electron – and show how this is represented in equations. 2) Be able to describe the substitution of alkanes using ultraviolet radiation, by Cl2 and Br2, to form halogenoalkanes. 3) Describe how homolytic fission leads to the mechanism of radical substitution in alkanes (initiation, propagation and termination reactions). 4) Explain the limitations of radical substitution in synthesis – arising from further substitution and formation of a mixture of products. Prac 13 Alkenes 1) State that alkenes and cycloalkenes are unsaturated hydrocarbons. 2) Describe the overlap of adjacent porbitals to form a bond 3) Explain the trigonal planar shape around each carbon in the C=C of alkenes. Prac 14 Addition 1) describe addition reactions of reactions of alkenes alkenes, with conditions – a) with hydrogen, b) halogens, c) hydrogen halides, d) steam to form alcohols. 2) Define an electrophile as an electron pair acceptor 3) Describe how heterolytic fission leads to the mechanism of electrophilic addition in alkenes Prac 15 Iodine and bromine tests for unsaturation. Polymers from alkenes 1) Describe the addition polymerization of alkenes 2) Deduce the repeat unit of an addition polymer obtained from a given monomer. 3) Identify the monomer that would produce a given section of an addition polymer. Prac 16 + 17 Industrial importance of alkenes 1) Outline the use of alkenes in the industrial production of organic compounds – manufacture of margarine by catalytic hydrogenation of unsaturated vegetable oils and a nickel catalyst. 2) Describe the formation of a range of polymers using unsaturated monomer units based on the ethene molecule (H2C=CHCl & F2C+CF2) Prac 18 Mod 2.2 Extraction of limonene from orange peel. Alcohol 1) Explain the water solubility and low volatility of alcohols with reference to hydrogen bonding. 2) Describe the industrial production of ethanol by fermentation of sugars, and the reaction of ethene with steam in the presence of an acid catalyst. 3) Outline the use of alcohols in i) ii) iii) alcoholic drinks as a solvent methanol as a petrol additive iv) methanol as a feedstock in the production of organic chemicals. Fermentation of glucose Prac 19 Reaction of alcohols 1) Be able to classify alcohols into primary, secondary and tertiary alcohols. 2) Be able to describe the combustion of alcohols. 3) Describe the oxidation of alcohols using Cr2O72- / H+ including: i) ii) oxidation of primary alcohols to from aldehydes and carboxylic acids. Oxidation of secondary alcohols to form ketones iii) Prac 20 The resistance to oxidation of tertiary alcohols Oxidation of ethanol to aldehyde and carboxylic acid. Reaction of alcohols (II) 1) Describe the esterification of alcohols with carboxylic acids in the presence of an acid catalyst 2) Describe the elimination of H2O from alcohols in the presence of an acid catalyst and heat to form alkenes. Prac Preparation of esters on a test-tube scale. Elimination of water from cyclohexanol. 21-22 Halogenoalkan es 1) Define the term nucleophile as an electron pair donor 2) describe the hydrolysis of halogenoalkanes as a substitution reaction. 3) Describe the mechanism of nucleophilic substitution in the hydrolysis of primary halogenoalkanes with hot aqueous alkali. 4) Explain the rates of hydrolysis of primary halogenoalkanes in terms of the relative bond enthalpies of carbon-halogen bonds. Compare rates of hydrolysis of halogenoalkanes Prac 22 Uses of halogenoalkan es 1) describe the uses of chloroethene and tetrafluoroethene to produce the plastics PVC and PTFE 2) Explain the reasons for using CFCs and the environmental damage caused by them 3) Describe the role of green chemistry in minimising damage to the environment by promoting biodegradable alternatives to CFCs (HCFCs, CO2 as a blowing agent for expanded polymers). Prac 23 Infrared spectroscopy 1) State that absorption of infrared radiation causes covalent bonds to vibrate. 2) Identify alcohols, aldehydes, ketones and carboxylic acids from their infrared spectrum. 3) State that modern breathalysers measure ethanol in the breath using infrared spectroscopy. Prac 24 - 25 Mass spectrometry 1) Describe mass spectrometry a method to determine relative isotopic masses, and identifying elements (for example on the Mars probe / measuring pollution – eg lead) 2) Interpret mass spectra of elements in terms of isotopic abundances. 3) Interpret mass spectra of organic compounds: i) ii) iii) to identify molecular mass identify major fragment ions predict structures from peaks 4) explain that a mass spectrum is a fingerprint for the molecule that can be identified by computers (and database library) Prac 26 Mod 2.3 http://riodb01.ibase.aist.go.jp/sdbs/cgibin/cre_index.cgi?lang=eng Enthalpy changes 1) Explain that some chemical reactions are accompanied by enthalpy changes – and can be exothermic (H negative) or endothermic (H positive). 2) Describe the importance of oxidation as an exothermic process in the combustion of fuels and the oxidation of carbohydrates. 3) Describe that endothermic processes require an input of heat energy, eg thermal decomposition of calcium carbonate. 4) Construct a simple enthalpy profile diagram for a reaction to show the difference in the enthalpy of the reactants compared with products Prac 27 Direct enthalpy changes of reaction for simple reactions: Zn + CuSO4 (exo); NaHCO3 + citric acid (endo); NaOH + HCl (exo). Enthalpy changes (II) 1) Explain qualitatively, using enthalpy profile diagrams, the term activation energy 2) Define and use the terms: standard conditions (298K and 100kPa), enthalpy change of reaction, enthalpy change of formation, and enthalpy change of combustion. 3) Calculate enthalpy changes from appropriate experimental results directly, including use of the relationship: energy change = mcT Prac Enthalpy change of combustion of alcohols 28 Bond enthalpies 1) Explain exothermic and endothermic reactions in terms of enthalpy changes associated with breaking and making bonds. 2) Define the use of term average bond enthalpy (positive H – bond breaking of one mole of bonds) 3) Calculate an enthalpy change of reaction from average bond enthalpies. Prac 29+30 Direct enthalpy changes of reaction for simple reactions: Zn + CuSO4 (exo); NaHCO3 + citric acid (endo); NaOH + HCl (exo). Hess’ law and enthalpy cycles 1) Use Hess’ law to construct enthalpy cycles and carry out calculations to determine enthalpy change of reaction from enthalpy changes for combustion. 2) Use Hess’ law to construct enthalpy cycles and carry out calculations to determine enthalpy change of reaction from enthalpy changes for formation. 3) Use Hess’ law to construct enthalpy cycles and carry out calculations to determine enthalpy change of reaction from an unfamiliar enthalpy cycle. Prac 31 Indirect enthalpy change of reaction: 2KHCO3 → K2CO3 + H2O + CO2 indirectly using HCl. Collision theory 1) describe qualitatively, in terms of collision theory, the effect of concentration changes on the rate of a reaction; 2) explain why an increase in the pressure of a gas, increasing its concentration, may increase the rate of a reaction involving gases; Prac 32 - 33 Rate graphs for gas products, eg CaCO3 + HCl; Mg + HCl Catalysts 1) State that a catalyst speeds up a reaction without being consumed by the overall reaction. 2) Explain that catalysts: i) ii) iii) iv) affect the conditions that are needed, usually lowering temperatures and reducing energy demand (lower CO2 and fossil fuels) allow scientists to use different reactions with better atom economy are often enzymes – and work at room temperature and pressures are of great economic importance using 4 examples. 3) Explain, using enthalpy profile diagrams, how a catalyst allows a reaction to follow a different route with a lower activation energy (increasing reaction rate) Prac 34 Boltzmann distribution 1) Explain qualitatively the Boltzmann distribution, and its relationship with the activation energy. 2) Describe qualitatively, using the Boltzmann distribution, the effect of temperature changes on the proportion of molecules exceeding the activation energy and rate of reaction. 3) Interpret how catalysts affect the Boltzmann distribution. Prac 35 Dynamic Equilibrium 1) State le Chatelier’s principle 2) Explain that a dynamic equilibrium exists when the rate of forward reaction is equal to the rate of the reverse reaction. 3) Apply le Chateliers’s principal to deduce the effect of changes in temperature, concentration and pressure on a homogeneous system in equilibrium 4) Explain (using data) the importance in the chemical industry of a compromise between chemical equilibrium and reaction rates. Prac Changing equilibrium position with heat: [Cu(H2O)6]2+ ⇌ CuCl42Changing equilibrium position with concentration: Fe3+ and SCN–