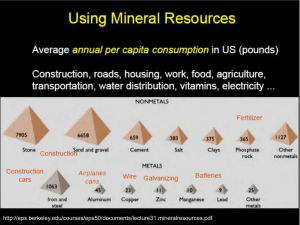
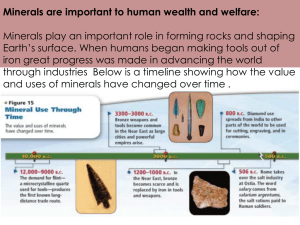
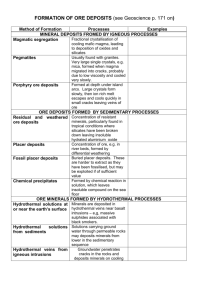
Soil chemistry, diagenesis
advertisement

Soil • soil - (i) The unconsolidated mineral or organic material on the immediate surface of the earth that serves as a natural medium for the growth of land plants. (ii) The unconsolidated mineral or organic matter on the surface of the earth that has been subjected to and shows effects of genetic and environmental factors of: climate (including water and temperature effects), and macro- and microorganisms, conditioned by relief, acting on parent material over a period of time. A product-soil differs from the material from which it is derived in many physical, chemical, biological, and morphological properties and characteristics. Soil Components • • • • • Minerals Organic Material Microorganisms Plants Gas – CO2, CH4, H2S, etc. • Water • Soil fabric – spatial arrangement of these things • Liquid – 1-35% • Chelates – organics that bond metals strongly, solubilizing them (bidentate or polydentate = 2 or 2+ bonds to the metal) – Equilibrium description? Chelators • These are key organic compounds which SIGNIFICANTLY affect how much metals get into water and how they can be transported: Cu solubility: • Cu2+ + EDTA Cu(EDTA) • 2Cu+ + O2- = Cu2O log K = 18 log K = -15 Soil stratigraphy • Soil layers, or Horizons, lettered OAEBCR • O = organic layer = plant fibers, high organics, leafy • A = topsoil = minerals + organics • E = leached layer = minerals leached, low organics • B = accumulation zone = leached and carried down, lots of clays • C = Parent material – partially weather original minerals • R = bedrock Diagenesis • Process of turning sediments into sedimentary rocks water-rock interactions precipitating minerals • Water is pores of sediments – ‘fresh’ muds can be >80% water… – Water can be trapped at time of deposition, transported in, or evolved from dehydration reactions of hydrous minerals • Also can be significant organic matter – Drive redox reactions – reduce Fe3+, Mn4+, SO42-… Diagenesis • Muds are compacted to shales – water is expelled, though up to 30% H2O can remain associated with clays even at 1 km depth • Minerals from water and changing conditions clays, sulfides, siicates, carbonates Clay Geochemistry • Clays can have significant chemical substitution, they undergo phase transitions as diagenesis proceeds • Illites Smectites in shales for example Al2Si4O10(OH)2*nH2O + KAlSi3O8 KAl2(AlSi3)O10(OH2) + 4 SiO2(aq) + n H2O Sandstone Diagenesis • Sandy sediments have high permeability, meaning water flows through them faster • The water brings ions, precipitation of calcite and silica occurs – WHY? • These minerals cement the sediments • Silica becomes a more important cementing material at high T • Pressurized pockets can become more concentrated, when the pressure is released they are instantly supersaturated… Carbonate Diagenesis • Aragonite and Mg-rich calcite are the major phases associated with shallow sedimentary carbonates • Dolomite problem: Dolomite is not the first thing to form typically from a water, why are units of calcite so extensively dolomitized? • Reaction requires a higher Mg/Ca ratio – occurring perhaps in sabka (supratidal pools) environments, or at seawater-meteoric water interfaces – where calcite is undersaturated but dolomite is supersaturated • How do these ions get to these places and form cement? • Transport through water… • Diffusion and advection account for the movement of ions Economic Geology • Understanding of how metalliferous minerals become concentrated key to ore deposits… • Getting them out at a profit determines where/when they come out Ore Deposits • Economic concentrations of materials are ores – combination of economics and geochemistry… • Geochemically we are looking at processes that concentrate ores to a very high degree – Magmatic differentaition – Weathering processes – Hydrothermal water/rock interactions *water is especially important at causing this concentration!! Gold Au • Distribution of Au in the crust = 3.1 ppb by weight 3.1 units gold / 1,000,000,000 units of total crust = 0.00000031% Au • Concentration of Au needed to be economically viable as a deposit = few g/t 3 g / 1000kg = 3g/ 1,000,000 g = 0.00031% Au • Need to concentrate Au at least 1000-fold to be a viable deposit • Rare mines can be up to a few percent gold (extremely high grade)! Ore minerals • Minerals with economic value are ore minerals • Minerals often associated with ore minerals but which do not have economic value are gangue minerals • Key to economic deposits are geochemical traps metals are transported and precipitated in a very concentrated fashion – Gold is almost 1,000,000 times less abundant than is iron Water-rock interactions • To concentrate a material, water must: – Transport the ions – A ‘trap’ must cause precipitation in a spatially constrained manner • Trace metals which do not go into igneous minerals easily get very concentrated in the last bit of melt • Leaching can preferentially remove materials, enriching what is left or having the leachate precipitate something further away Ore deposit environments • Magmatic – Cumulate deposits – fractional crystallization processes can concentrate metals (Cr, Fe, Pt) – Pegmatites – late staged crystallization forms pegmatites and many residual elements are concentrated (Li, Ce, Be, Sn, and U) • Hydrothermal – Magmatic fluid - directly associated with magma – Porphyries - Hot water heated by pluton – Skarn – hot water associated with contact metamorphisms – Exhalatives – hot water flowing to surface – Epigenetic – hot water not directly associated with pluton Hydrothermal Ore Deposits • Thermal gradients induce convection of water – leaching, redox rxns, and cooling create economic mineralization Metal Sulfide Mineral Solubility • Problem 1: Transport of Zn to ‘trap’: ZnS + 2 H+ + 0.5 O2 = Zn2+ + S2- + H2O log K 9.57 log [ Zn 2 ] f S 2 [ H 2 O] [ H ]2 f O02.5 [ ZnS ] Need to determine the redox state the Zn2+ would have been at equilibrium with… What other minerals are in the deposit that might indicate that? define approximate fO2 and fS2values and compute Zn2+ conc. Pretty low Zn2+ • Must be careful to consider what the conditions of water transporting the metals might have been how can we figure that out?? • What other things might be important in increasing the amount of metal a fluid could carry? More metal a fluid can hold the quicker a larger deposit can be formed… • How about the following: ZnS + 2 H+ + 0.5 O2 + Cl- = ZnCl+ + S2- + H2O log K 16.6 log [ ZnCl ] f S 2 [ H 2 O] [ H ]2 f O02.5 [ ZnS ][Cl ] Compared to log K 9.57 log [ Zn 2 ] f S 2 [ H 2 O] [ H ]2 f O02.5 [ ZnS ] That is a BIG difference… Geochemical Traps • Similar to chemical sedimentary rocks – must leach material into fluid, transport and deposit ions as minerals… • pH, redox, T changes and mixing of different fluids results in ore mineralization • Cause metals to go from soluble to insoluble • Sulfide (reduced form of S) strongly binds metals many important metal ore minerals are sulfides! Piquette Mine • 1-5 nm particles of FeOOH and ZnS – biogenic precipitation •Tami collecting samples cells ZnS Piquette Mine – SRB activity • At low T, thermochemical SO42- reduction is WAY TOO SLOW – microbes are needed! • ‘Pure’ ZnS observed, buffering HS- concentration by ZnS precipitation Fluid Flow and Mineral Precipitation • monomineralic if: – flux Zn2+ > HS- generation – i.e. there is always enough Zn2+ transported to where the HS- is generated, if • sequential precipitation if: – Zn2+ runs out then HS- builds until PbS precipitates y Pb2+ ZnS ZnS ZnS PbS x Zn2+ z HS- generated by SRB in time t Model Application • Use these techniques to better understand ore deposit formation and metal remediation schemes Sequential Precipitation Experiments • SRB cultured in a 125 ml septum flask containing equimolar Zn2+ and Fe2+ • Flask first develops a white precipitate (ZnS) and only develops FeS precipitates after most of the Zn2+ is consumed • Upcoming work in my lab will investigate this process using microelectrodes where observation of ZnS and FeS molecular clusters will be possible! Ore deposit environments • Sedimentary – Placer – weathering of primary mineralization and transport by streams (Gold, diamonds, other) – Banded Iron Formations – 90%+ of world’s iron tied up in these (more later…) – Evaporite deposits – minerals like gypsum, halite deposited this way – Laterites – leaching of rock leaves residual materials behind (Al, Ni, Fe) – Supergene – reworking of primary ore deposits remobilizes metals (often over short distances) Ore Deposit Types I • • • • • • • • • • • • • • Placer uranium gold Stratiform phosphate Stratiform iron Residually enriched deposit Evaporites Exhalative base metal sulphides Unconfornity-associated uranium Stratabound clastic-hosted uranium, lead, copper Volcanic redbed copper Mississippi Valley-type lead-zinc Ultramafic-hosted asbestos Vein uranium Arsenide vein silver, uranium Lode Gold Ore Deposit Types II • • • • • • • • • • • • • Clastic metasediment-hosted vein silver-lead-zinc Vein Copper Vein-stockwork tin, tungsten Porphyry copper, gold, molybdenum, tungsten, tin, silver Skarn deposits Granitic pegmatites Kiruna/Olympic Dam-type iron, copper, uranium, gold, silver Peralkaline rock-associated rare metals Carbonatite-associated deposits Primary diamond deposits Mafic intrusion-hosted titanium-iron Magmatic nickel-copper-platinum group elements Mafic/ultramafic-hosted chromite Metamorphism • At temperatures greater than 200-300°C but less than melting, reactions changing the mineralogy and fabric of rock are metamorphic • P-T changes with burial, tectonic stresses, geothermal gradient differences, etc…. • Prograde – ‘forward’ direction – rxns occurring with increasing P-T • Retrograde – ‘back’ direction – rxns occurring with decreasing P-T Phase Relations • Rule: At equilibrium, reactants and products have the same Gibbs Energy – For 2+ things at equilibrium, can investigate the P-T relationships different minerals change with T-P differently… • For DGR = DSRdT + DVRdP, at equilibrium, DG0, rearranging: DS R P T DG 0 DVR Clausius-Clapeyron equation DS R P T DG 0 DVR V = Vº(1-bDP) S S P S 0 dP S 0 VdP P T P2 P2 P1 DSR change with T or P? DCP DS R D T P T DS R VR D V T P R T P1 b S 0 V 0 DP ( P22 P12 2 DV for solids stays nearly constant as P, T change, DV for liquids and gases DOES NOT • Solid-solid reactions linear S and V nearly constant, DS/DV constant + slope in diagram • For metamorphic reactions involving liquids or gases, volume changes are significant, DV terms large and a function of T and P (and often complex functions) – slope is not linear and can change sign (change slope + to –) DS R P T DG 0 DVR