CI 13.2 – Alcohols and Ethers
advertisement

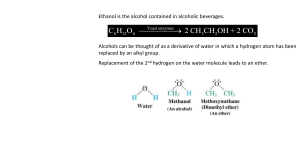
CI 13.2 – Alcohols and Ethers (C) JHUDSON 2005 Draw two different structures with the molecular formula C2H6O Remember carbon bonds to 4 other atoms, oxygen bonds to 2 Ethanol (an alcohol) Methoxymethane (an ether) Alcohols All alcohols contain the hydroxyl functional group -OH and have similar chemical properties Ethanol can be represented any of these ways; Naming Alcohols Full structure formula Skeletal formula Name Ethanol Propan-1-ol Propan-2-ol Full structure formula Skeletal formula Name Butan-1-ol Pentan-1-ol Butan-2-ol Pentan-3-ol Physical Properties of Alcohols Like water molecules, alcohols are polar due to the electronegativity of the oxygen atom. Alcohol molecules can hydrogen bond to each other and to water molecules. This explains their solubility in water. At room temperature ethanol is a liquid whilst ethane is a gas. Can you explain why? Ethanol has a higher boiling point because the attractive forces between its molecules are greater. The intermolecular bonds must be broken to form a gas. Alcohol molecules can hydrogen bond to water molecule so alcohols are soluble in water. For bigger alcohol molecules the influence of the OH group becomes less. So longer chain alcohols are less soluble. Ethers Methoxyethane Methoxymethane We can think of ethers as like water molecules with both hydrogen atoms replaced by alkyl groups. No hydrogen bonding can occur and the molecules are only slightly polar so boiling pts are similar to alkanes with similar mass.