Category 1
advertisement

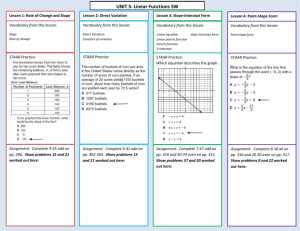
STAAR Reporting Category 1 Matter & Energy Middle School Science Science STAAR Need to Know STAAR Need to Know 1 Atomic Theory Atoms are building blocks of elements Atoms in each unique element are the same. (Ex: Every oxygen element has the same # of protons) Atoms are different from atoms of other elements (Ex: Hydrogen has a different # of protons than oxygen) Two or more different atoms bond in simple ratios to form compounds (Ex: Hydrogen & oxygen can chemically combine to form water- H2O ) STAAR Need to Know 2 STAAR Need to Know 3 STAAR Need to Know 4 Atoms and Elements STAAR Need to Know 5 Atomic Particle Charges Protons Positive + Neutrons Neutral = Electrons Negative STAAR Need to Know 6 Location of Subatomic Particles 10-13 cm electrons protons nucleus neutrons 10-8 cm STAAR Need to Know 7 Location of Atomic Particles Inside the Nucleus Outside the Nucleus- in electron cloud Protons with a Positive charge AND Neutrons with a Neutral charge –that means they do not have a charge Electrons with a Negative charge Did you know electrons are very, very small and move very, very fast??? STAAR Need to Know 8 Periodic Squares 11 Na 22.99 The periodic square for an element can tell you lots of information about that atom. Atomic number = # of protons Atomic number = # of electrons That means in a neutral (uncharged) atom the # of protons and electrons are always the SAME!!!! STAAR Need to Know 9 Atomic Mass on the Periodic Table Atomic Number or Protons 11 Symbol Atomic Mass or Protons + neutrons Na 22.99 STAAR Need to Know 10 Atomic number Atomic number = # of Protons STAAR Need to Know 11 Mass Number The number of protons and neutrons in an atom STAAR Need to Know 12 Number of Electrons A balanced atom is neutral The net overall charge is zero Number of protons = Number of electrons Atomic number = Number of electrons STAAR Need to Know 13 STAAR Need to Know 14 STAAR Need to Know 15 STAAR Need to Know 16 Elements with similar properties are placed in the same group in the periodic table. The stair-step line separates the elements into metals and nonmetals. STAAR Need to Know 17 Metals & Nonmetals STAAR Need to Know 18 Element Chlorine-35 Iron-56 Magnesium-24 Lead-208 Nitrogen-14 Protons Neutrons Electrons 17 26 12 82 7 18 30 17 26 2 elements with similar properties F and Br Ru and Os 12 12 Ca and Sr 126 82 Sn and Ge 7 7 P and As19 STAAR Need to Know Molecules & Compounds STAAR Need to Know 20 STAAR Need to Know 21 Mixtures Combination of two or more pure substances. Substances are mixed together but have not reacted to form any new molecules. Example- Sugar (a compound) dissolves in water (a compound) to form a mixture. The molecules of sugar and water do not change chemically. They just become mixed together. STAAR Need to Know 22 Properties of matter Physical properties – can be observed without changing the substance into a different substance. STAAR Need to Know 23 Chemical Properties Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chemical change has occurred. Hydrogen gas and oxygen gas each have a different set of properties. Substances change into different substances through STAAR Need to Know chemical reactions. 24 Chemical Reactions Substances change into other substances in chemical reactions. The atoms in the original substance are rearranged. The bonds in the original substance may be broken and new bonds may be formed between different atoms. This produces one or more new substances that may be either pure elements or compounds. The products of a chemical reaction always have difference chemical and physical properties than the original substance(s). STAAR Need to Know 25 Evidence of a Chemical Reaction a change in odor, a change in color, a solid precipitate is formed, a gas is formed, there is either absorption or release of heat STAAR Need to Know 26 STAAR Need to Know 27 STAAR Need to Know 28 Chemical Equations STAAR Need to Know 29 STAAR Need to Know 30