Primary hemostasis - Cal State LA
advertisement

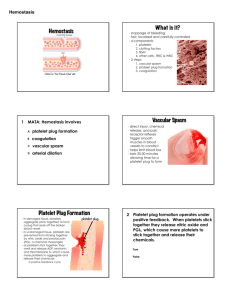
HEMOSTASIS Primary hemostasis HEMOSTASIS Hemostasis The process by which the body stops bleeding upon injury and maintains blood in the fluid state in the vascular compartment Process is rapid and localized HEMOSTASIS The primary players in hemostasis include Blood vessels Platlets Plasma proteins Coagulation proteins – involved in clot formation Fibrinolysis – involved in clot dissolution Serine protease inhibitors Other minor players include Kinin system Complement system HEMOSTASIS Defects In blood vessels, platlets or serum proteins can be corrected by utilization of the other 2 players In 2 of the 3 players results in pathologic bleeding Blood Vessels Platlets Plasma Proteins HEMOSTASIS Hemostasis can be divided into two stages Primary hemostasis Response to vascular injury Formation of the “platelet plug” adhering to the endothelial wall Limits bleeding immediately Secondary Hemostasis Results in formation of a stable clot Involves the enzymatic activation of coagulation proteins that function to produce fibrin as a reinforcement of the platelet plug Gradually the stable plug will be dissolved by fibrinolysis FORMATION OF A STABLE PLUG VASCULAR SYSTEM Smooth and continuous endothelial lining is designed to facilitate blood flow Intact endothelial cells inhibit platelet adherence and blood coagulation Injury to endothelial cells promotes localized clot formation Vasoconstriction Narrows the lumen of the vessel to minimize the loss of blood Brings the hemostatic components of the blood (platelets and plasma proteins) into closer proximity to the vessel wall Enhances contact activation of platlets Von Willebrand factor Collagen fibers Platlet membrane glycoprotein Ib Activated platlets enhance activation of coagulation proteins PRIMARY HEMOSTASIS Platelets Interact with injured vessel wall Interact with each other Produce the primary hemostatic plug Primary platelet plug Fragile Can easily be dislodged from the vessel wall PLATELETS Platelets Small, anucleated cytoplasmic fragments Released from megakaryocytes in the BM Megakaryocyte proliferation is stimulated by thrombopoietin (TPO) Normal platlet count is 150-400 x 109/L Survive 9-12 days Nonviable or aged platelets removed by spleen & liver 2/3 of platelets circulate in the peripheral blood 1/3 are sequestered in the spleen Humoral factor Produced primarily by liver, kidney, spleen, BM Produced at a relatively constant rate These 2 pools are in equilibrium and constantly exchanging Spontaneous hemorrhaging occurs when platlet count gets below 10 x 109/L PLATLETS MATURE MAGAKARYOCYTE PLATLET RELEASE PLATLET FUNCTION Platlets function to Provide negatively charged surface for factor X and prothrombin activation Release substances that mediate vasoconstriction, platlet aggregation, coagulation, and vascular repair Provide surface membrane proteins to attach to other platlets, bind collagen, and subendothelium PLATELETS Are the primary defense against bleeding Circulate in resting state Have minimal interaction with other blood components or the vessel wall Morphology of resting platelet is smooth, discoid When stimulated by endothelial damage, platlets become activated and they Become round and ‘sticky’ Build a hemostatic plug Provide reaction surface for proteins that make fibrin Aid in wound healing Platlet activation and plug formation involves Adhesion Shape change Secretion Aggregation ADHESION Damage to endothelium exposes blood to the subepithelial tissue matrix with adhesive molecules Platlet receptor GPIb binds to subendothelium collagen fibers through von Willebrand’s factor (vWF) Platlet adherence stops the initial bleeding SHAPE CHANGE Following vessel injury and platlet exposure to external stimuli, platlets change shape from circulating discs to spheres with pseudopods Shape change is mediated by an increase in cytosolic calcium Exposure of platlet membrane phospholipids promotes the assembly of vitamin-K dependent factors on the platlet membrane surface Activated platlets adhere to exposed collagen CHANGE IN PLATLET SHAPE AGGREGATION Platlet-to-platlet interaction Begins 10-20 seconds after vascular injury and platlet adhesion Requires dense granule release from the adhering platlets Requires Ca++ and ATP Requires fibrinogen and fibrinogen receptors GPIIb and IIIa Mechanism: ADP released from platlet cytoplasm upon adherence induces exposure of fibrinogen receptors GPIIb and IIIa Fibrinogen binds to the exposed GPIIb and IIIa Extracellular Ca++-dependent fibrinogen bridges form between adjacent platlets, thereby promoting platlet aggregation This is primary or reversible aggregation Secondary aggregation begins with the release of dense granules Secondary aggregation is considered irreversible SECRETION Secondary aggregation begins with platlet secretion of dense granules Dense granules contain large amounts of ADP ADP binds to the platlet membrane triggering the synthesis and release of TXA2 The release of large amounts of ADP combined with TXA2 amplifies the initial aggregation of platlets into a large platlet mass FORMATION OF PRIMARY HEMOSTATIC PLUG PLATELETS AND SECONDARY HEMOSTASIS Primary platelet plug is Unstable and easily dislodged Secondary hemostasis Fibrin formation stabilizes the platelet plug Proteins interact to form fibrin assemble on negatively charged membrane phospholipids of activated platelets