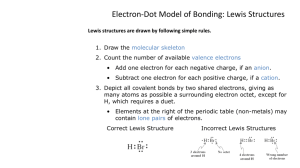
Section 8.2
Exceptions to the octet rule
Electronegativity
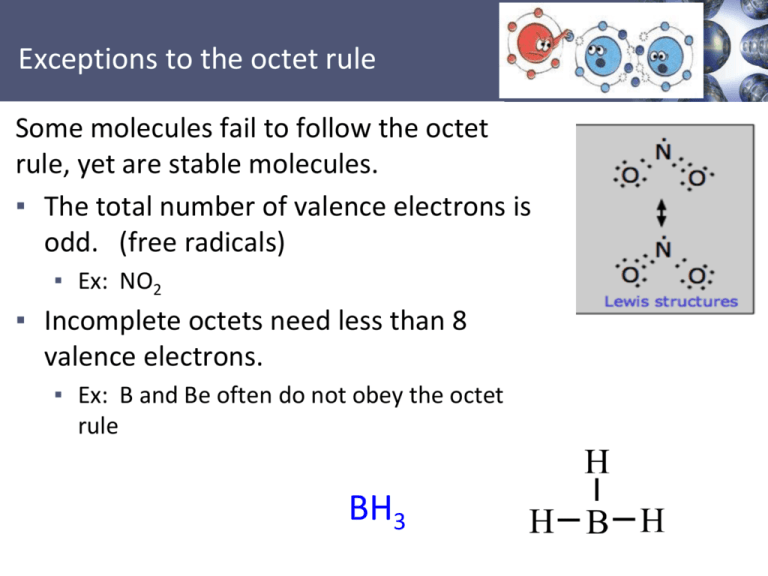
Some molecules fail to follow the octet
rule, yet are stable molecules.
▪ The total number of valence electrons is
odd. (free radicals)
▪ Ex: NO2
▪ Incomplete octets need less than 8
valence electrons.
▪ Ex: B and Be often do not obey the octet
rule
BH3
Copyright © Cengage Learning. All rights reserved
Section 8.2
Electronegativity
▪ Expanded octets exceed the octet rule. Extra electrons
are placed on the central atom.
▪ Example:
SF4 = 34e–
Copyright © Cengage Learning. All rights reserved
AsBr5 = 40e–
Section 8.2
Resonance
Electronegativity
Resonance occurs when more than one valid Lewis
structure can be written for a particular molecule.
Example: NO3–
Copyright © Cengage Learning. All rights reserved
Section 8.2
Electronegativity
▪ Actual structure is an average or hybrid of the resonance
structures.
▪ Electrons are really delocalized – they can move around
the entire molecule.
▪ It’s as if the structures were changing infinitely fast, so
that electrons and charges were everywhere at once.
Copyright © Cengage Learning. All rights reserved
Section 8.2
Electronegativity
Problem:
Draw all resonance structures for OCNWhich resonance structure is correct?
When nonequivalent resonance structures exist, the
most likely resonance structure is the one with formal
charges closest to zero.
Section 8.2
Formal
Charge
Electronegativity
Formal Charge
▪Formal charge represents the difference between
the number of valence electrons an atom possesses
in its free state and the number assigned to it in a
given Lewis structure.
▪The number of valence electrons assigned to an
atom in the bonded state can be found by counting
all of the electrons belonging exclusively to that
atom (i.e., non-bonding electrons) and one-half of
the electrons in the bonds to it.
Chapter
8
Formal Charge
The formula:
The formal charge is useful to determine the
stabilities of resonance forms or in establishing the
best arrangement of atoms in a molecule where the
central atom is not immediately apparent
Determine
Chapter 8 the formal charge on each atom
Chapter
8
Formal Charge
Use formal charge to determine the best
structure for OCN-
The first structure is the most unlikely due to
the higher formal charges
When a tie occurs, use electronegativity.
Since oxygen has a higher electronegativity than
nitrogen, the last structure is the most likely
Chapter
8
Formal Charge
Sample problem: Choose the most likely structure:
B is the most likely. The f.c. are close to 0, and the highly
electronegative oxygen atom is a -1.
A is unlikely because of the large positive formal charge on
Cl.
Structure C is unlikely because the least electronegative
element (Cl) has a negative f.c. while the most
electronegative element in the ion (O) has a f.c. of zero.
Chapter
8
Formal Charge
Example: Draw the Lewis structure for NO2. It
does not follow the octet rule.
Which structure is the best Lewis structure
Section 8.2
Electronegativity
End lesson 3