chapter 1 - UniMAP Portal
advertisement

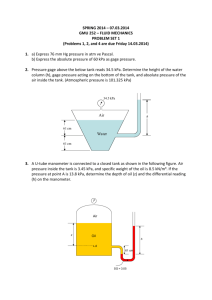
ERT 206/4 THERMODYNAMICS SEM 2 (2011/2012) The name thermodynamics stems from the Greek words therme (heat) and dynamis (power). Energy: Conservation of energy principle: During an interaction, energy can change from one form to another but the total amount of energy remains constant. The ability to cause changes. The first law of thermodynamics: Energy cannot be created or destroyed; it can only change forms. The second law of thermodynamics: It asserts that energy has quality as well as quantity, and actual processes occur in the direction of decreasing quality of energy. Heat flows in the direction of decreasing temperature The first law of thermodynamics Power plant Aircraft and spacecraft Car Human body Wind turbines Refrigeration systems Air conditioning systems Boats Solar hot water systems Any physical quantity can be light characterized by dimensions The magnitudes assigned to the light dimension are called units L of Electric current AmountL of light Amount of matter Primary/ fundamental light dimensions Temperature light Length light Mass light Time light English light system Primary dimensions and their units in SI International light system (SI) Secondary/ derived light dimensions Velocity light Standard prefixes in SI units Volume light Energy light Force light Mass, lightm Total volume, Vt light No. oflight moles, n Represent the size of alight system m nM Mass light No. of moles Molecular weight No. of moles Mass m n light M Molecular weight Specific volume: Vt V m or V t Vm Molar volume: Vt V n or V t Vn m=1 kg a=1 m/s2 m=32.174 lbm Definition: F light ma light a=1 ft/s2 Mass F=1 N F=1 lbf Acceleration light a mass of 1 kg or Force required to accelerate 32.174 lbm at a rate of 1 m/s2 or 1 ft/s2. Mass Definition: W mg light Weight is gravitational force applied to a body Local gravitational acceleration light g = 9.807 m/s2 = 32.174 ft/s2 English system light Pound-force (lbf) Unit in many European countries light Kilogram-force (kgf) 1 N = 1 kg. m/s2 light 2 = 4.44822 N 1 lbf = 32.174 lbm.ft/s 1 kgf = 9.807 N SI light Newton (N) • System: A quantity of matter or a region in space chosen for study. • Surroundings: The mass or region outside the system • Boundary: The real or imaginary surface that separates the system from its surroundings. • The boundary of a system can be fixed or movable. • Systems may be considered to be closed or open. • Closed system (Control mass): A fixed amount of mass, and no mass can cross its boundary. • Open system (control volume): Both mass and energy can cross the boundary of a control volume. • Device: compressor, turbine, or nozzle. • Control surface: The boundaries of a control volume. It can be real or imaginary. An open system (a control volume) with one inlet and one exit. 8 Commonly measured with liquid-in-glass thermometer, wherein the liquid expands when heated Boiling point of pure water at light pressure standard atmospheric Freezing point of water saturated with air at standard light atmospheric pressure Lower limitlight of temperature Relations among light temperature scales Comparison of light magnitude of various temperature units Electric power generation by a wind turbine A school is paying $0.09/kWh for electric power. To reduce its power bill, the school install a wind turbines with a rated power of 30 kW. If the turbine operates 2200 hours per year at the rated power, determine the amount of electric power generated by the wind turbine and the money saved by the school per year. a) Determine the total energy b) Determine the money saved a) Determine the total energy Total energy = (Energy per unit time) (Time interval) = (30 kW) (2200 h) = 66, 000 kWh b) Determine the money saved Money saved = (Total energy) (Unit cost of energy) = (66,000 kWh) ($0.09/kWh ) = $5940 Convert your answer of total energy in kJ. Total energy = 66,000 kWh 3600 s 1 kJ/s 1h 1 kW = 2.38 X 108 kJ The weight of one pound-mass Using unity conversion ratios, show that 1.00 lbm weighs 1.00 lbf on earth. W = mg = 1.00 lbm 32.174 ft/s2 1 lbf 32.174 lbm.ft/s2 = 1.00 lbf Expressing Temperature Rise in Different Units During a heating process, the temperature of a system rises by 10 ⁰C. Express this rise in temperature in K, ⁰F and R. Δ T(K) = Δ T(⁰C) = 10 K Δ T(R) = 1.8 Δ T(K) = (1.8) (10) = 18 R Δ T(⁰F) = Δ T(R) = 18 ⁰F A normal force exerted by a fluid per unit area light Some basic pressure gages. • Absolute pressure: The actual pressure at a given position. It is measured relative to absolute vacuum (i.e., absolute zero pressure). • Gage pressure: The difference between the absolute pressure and the local atmospheric pressure. Most pressure-measuring devices are calibrated to read zero in the atmosphere, and so they indicate gage pressure. • Vacuum pressures: Pressures below atmospheric pressure. The pressure of a fluid at rest increases with depth (as a result of added weight). In a room filled with a gas, the variation of pressure with height is negligible. Pressure in a liquid at rest increases linearly with distance from the free surface. The pressure is the same at all points on a horizontal plane in a given fluid regardless of geometry, provided that the points are interconnected by the same fluid. Pascal’s law: The pressure applied to a confined fluid increases the pressure throughout by the same amount. light Lifting of a large weight by a small force by the application of Pascal’s law. It is commonly used to measure small and moderate pressure differences. A manometer contains one or more fluids such as mercury, water, alcohol, or oil. The basic manometer. Measuring the pressure drop across a flow section or a flow device by a differential manometer. In stacked-up fluid layers, the pressure change across a fluid layer of density and height h is gh. • Atmospheric pressure is measured by a device called a barometer; thus, the atmospheric pressure is often referred to as the barometric pressure. • A frequently used pressure unit is the standard atmosphere, which is defined as the pressure produced by a column of mercury 760 mm in height at 0°C (Hg = 13,595 kg/m3) under standard gravitational acceleration (g = 9.807 m/s2). The basic barometer. Absolute Pressure of a Vacuum Chamber A vacuum gage connected to a chamber reads 40 kPa at a location where the atmospheric pressure is 100 kPa. Determine the absolute pressure in the chamber. Pabs = Patm - Pvac = 100 - 40 = 60 kPa Measuring Pressure with a Manometer A manometer is used to measure the pressure in a tank. The fluid used has a specific gravity of 0.85, and the manometer column height is 55 cm, as shown in figure. If the local atmospheric pressure is 96 kPa, determine the absolute pressure within the tank. ρ SG(ρ H 2O ) (0.85)(100 0kg/m 3 ) 850 kg/m 3 P Patm ρgh = 96 kPa + 850 kg/m3 9.81 m/s2 = 100.6 kPa Determine the gage pressure in the tank. 0.55 m 1N 1 kPa 1 kg.m/s2 1000 N/m2 Measuring Pressure with a Multifluid Manometer The water in a tank is pressurized by air and the pressure is measured by a multifluid manometer as shown in the figure. The tank is located on a mountain at an altitude of 1400 m where the atmospheric pressure is 85.6 kPa. Determine the air pressure in the tank if h1 = 0.1 m, h2 = 0.2 m and h3 = 0.35 m. Take the densities of water, oil and mercury to be 1000 kg/m3, 850 kg/m3 and 13600 kg/m3, respectively. Ans: P1 = 130 kPa Measuring Atmospheric Pressure with a Barometer Determine the atmospheric pressure at a location where the barometric reading is 740 mmHg and the gravitational acceleration is g = 9.81 m/s2. Assume the temperature of mercury to be 10 ⁰C, at which its density is 13570 kg/m3. Ans: in unit kPa Ans: 98.5 kPa Work, energy and heat will be covered in other chapter! Work = Force Distance 1 J =light 1 N∙m 1 cal = 4.1868 J 1 Btu = 1.0551 kJ