Cyanide and Methemoglobinemia
advertisement

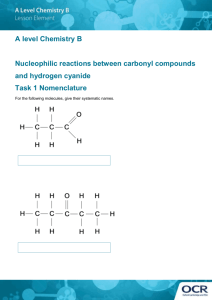
Cyanide and Methemoglobinemia Presented by: Dr. Aric Storck Preceptor: Dr. Ingrid Vicas Core Rounds February 20, 2003 Cyanide Cyanide Anion (CN-) solid and gaseous forms Important component of many industrial reactions mining - recover silver and gold from ores photographic - recovery of silver plastic manufacturing Naturally occurs in many plant products Tobacco, apricot pits Cyanide pollution 1997 - 4,513,410 cyanide released by top 100 polluters in USA Bhopal, 1984 worst industrial poisoning in history 25,000 kg methyl isocyanate and combustion products released into atmosphere 1,800 - 5,000 deaths 200,000 injuries Man carries his wife past the Union Carbide factory in Bhopal, India. Fumes from the factory killed her the previous day Source: Greenpeace Skulls from victims of the Union Carbide disaster in the Hamidia Hospital in Bhopal, India. Source: Greenpeace Cyanide … a potential disaster 500,000 hazardous materials shipments / day in the USA Average 12,115 hazardous material accidents per year (1990-1996) Large potential for significant industrial accident involving cyanide Cyanide and terrorism 1984 - 7 Chicago area residents killed after ingesting cyanide-laced Tylenol Cyanide gas precursors (cyanide salt + acid) found in Tokyo subway bathrooms following sarin gas attacks Cyanide believed to be involved in World Trade Center bombing (incinerated in attack) Cyanide and Fires Cyanide is a combustion product of plastic rugs silks furniture construction materials Cyanide and Fires Significant correlation between CO levels and CN- levels in fire victims estimated 35% of fire victims have toxic levels of cyanide Source: Sauer S, Keim M. Hydroxocobalamin: Improved public health readiness for cyanide disasters. Ann Emerg Med. June 2001; 37:635-641. Cyanide - Pathophysiology CN- has high affinity for metals Complexes with metallic cations at catalytic sites of several enzymes Binds ferric (3+) iron of mitochondrial cytochrome oxidase (cytochrome a-a3) cytochrome a-a3 – mediates transfer of electrons to molecular oxygen (final step in oxidative phosphorylation) Oxidative Phosphorylation Source: Ford’s Clinical Toxicology Blockade of oxidative phosphorylation Tissue anoxia Anaerobic metabolism Lactic acidosis Cyanide - Pathophysiology Other metabolic effects Less relevant (...because you die of anoxia first) Interferes with lipid metabolism Interferes with glycogen metabolism Cyanide - Poisoning Rapid absorption Respiratory tract Mucous membranes Slow absorption Skin GI tract Cyanide Poisoning - Inhalation Hydrogen cyanide Combustion of nitrogen containing polymers (vinyl, polyurethane, silk) Immediate onset of symptoms 50 ppm Symptoms after several hours Anxiety, SOB, palpitations, headache 100 ppm Death after 30 minutes 270 ppm Immediate coma, asystole, death Cyanide – Ingested Salts Symptoms within minutes Caustic – oral burns Smell of bitter almonds 50 mg – has been reported to cause death LD50 – 140-250 mg (untreated adult) Ingestion – Cyanide producing compounds Compounds require metabolic activation to produce cyanide Organic nitriles Cyanogenic glycosides eg: amygdalin – found in bitter almonds, apricot pits Hydrolyzed to CN in small bowel Not toxic if taken intravenously Acetonitrile (solvent in artificial nail remover) Oxidized by hepatic enzymes Delayed onset of symptoms (up to 24 hours) Cyanide - Dermal Exposure LD50 = 100 mg/kg Cyanide & Nitroprusside Deterioration in aqueous solutions releases cyanide Hydroxycobalamin and thiosulfate coinfusions used in critical care settings Chronic Cyanide Poisoning Clinical relevance controversial Cassava – contains linamarin (cyanogenic) Common food in many countries Some evidence that B12 deficiency, goiter, demyelinating diseases may be related Cyanide - Detoxification Naturally occurs in small quantities tobacco cassava Small amounts routinely cleared from body Cyanide - Detoxification Cyanide + thiosulfate = thiocyanate Enzymatically Rhodanase Beta-mercaptopyruvate-cyanide sulfur transferase Nonenzymatically Sulfane-albumin complex combines with cyanide Cyanide - Elimination Thiocyanate Relatively non-toxic Renal elimination (half life 2.5 days) Cyanide – Clinical Presentation Physiologic manifestations of hypoxia Metabolic acidosis Bradycardia Dyspnea CNS disturbances Normal pulse oximetry Cyanide – Clinical Presentation CNS Headaches Drowsiness Dizziness Seizures Coma Cyanide – Clinical Presentation Pulmonary Dyspnea Tachypnea Apnea Cyanide Poisoning - DDX Ingestion with altered LOC and acidosis sodium azide salicylates iron Beta-adrenergic antagonists cocaine isoniazid toxic alcohols Cyanide Poisoning - DDX Inhalational Exposures hydrogen sulfide carbon monoxide simple asphyxiants Cyanide – Clinical Presentation Cardiovascular Effects Hypertension Tachycardia Hypotension Bradycardia Asystole Cardiac collapse Laboratory Investigation Electrolytes Elevated anion gap (lactic acidosis) ABG Metabolic acidosis (lactic acidosis) Normal PO2 SaO2 Normal Laboratory Investigation AVO2 Decreased (decreased tissue oxygen utilization) Cyanide levels Not rapid enough for clinical utility Serum cyanide level Toxic = >0.5mg/L Fatal = >3.0 mg/L Erythrocyte cyanide level Normal = <1.9 uM/L (50ug/L) Fatal = > 40 uM/L (1mg/L) Laboratory Investigation Serum lactate – elevated ECG Sinus bradycardia Sinus tachycardia Cyanide Poisoning - Sequellae Directly related to severity of exposure and delay in treatment long term sequellae are those of hypoxia cerebral hypoxia / encephalopathy (common) Cyanide - Treatment Monitors IV access Administer 100% O2 Gastric lavage Indicated in very recent ingestion Activated charcoal (1g/kg) Cyanide Antidote Kit Manufacturer: Taylor Pharmaceuticals Cost: $317 USD Cyanide Antidote Kit Contents Amyl nitrite 0.3 ml x 12 Inhaled while IV access established Not necessary if immediate IV access Can be given in pre-hospital setting Sodium nitrite 300mg/10cc x 2 Sodium thiosulfate 12.5g/50cc x2 syringes, needles, tourniquet, stomach tube, instructions Cyanide Antidote Kit Instructions Crush and inhale one ampoule (0.3ml) of amyl nitrite q15-30 seconds until iv access achieved Rapid infusion sodium nitrite 300mg Infuse sodium thiosulfate 12.5g over 10 minutes Repeat sodium nitrite and thiosulfate infusion at half dose prn x 1 Caution Sodium nitrite infusion limited by hypotension Cyanide Antidote Kit - Mechanism Nitrites Therapeutic induction of methemoglobinemia NO2 + Hb = MHb Methemoglobin binds strongly to CN- and removes it from tissues CN- + MHB = cyanomethemoglobin cyanomethemoglobin relatively non-toxic Sodium Thiosulfate donates sulfur molecule to rhodanese (enzyme which catalyzes formation of thiocyanate) Na2S2O3 + HCN + O = HSCN Synergistic effect Oxygen Synergy of 100% O2 with nitrites/thiosulfate CAK - Children 0.33 mL/kg of 3% NaNO2 Adjust dose if anemic Hb 70 – 0.19mL/kg Hb 100 – 0.27mL/kg Hb 120 – 0.33mL/kg Hb 140 – 0.39mL/kg 1.65 mL/kg of 25% Na2S2O3 Cyanide Antidote Kit Effectiveness able to detoxify 20 lethal ingested doses in dogs effective even after respiratory arrest as long as no cardiac arrest Complications Hypotension Related to vasodilatory effects of nitrites Methemoglobinemia Death reported in asymptomatic cyanide poisonings (NB: only use CAK if symptomatic poisoning) Cyanide Antidote Kit Limitations MHb production prevents its use in unconfirmed cases not practical for smoke inhalation victims (bad idea to induce MHb when already high level of carboxyhemoglobin) many hospitals poorly supplied 81% of Tennessee hospitals unable to treat two 70 kg patients Cyanide – other antidotes Hyperbaric Oxygen No therapeutic effect Useful if concomitant CO inhalation Dicobalt edetate Widely used in UK Effective antidote with significant toxicity (esp. when cyanide not present) DMAP (4-dimethylaminophenol) Produces very rapid methemoglobinemia Used widely in Germany No more effective than sodium nitrite Less hypotension than sodium nitrite Linked with renal failure in animal models Hydroxycobalamin (vitamin B12a) Widely used in France Very effective and non-toxic precursor of B12 (cyanocobalamin) ideal choice for vegan victims of cyanide poisoning Recognized by FDA for cyanide poisoning Used in ICU settings to mitigate nitroprusside toxicity Reduces cyanide to cyanocobalamin B12a + CN- = B12 5g B12a will treat patients with up to 40 umol/L Low concentrations available in US mean very large quantities required Hydroxycobalamin (vitamin B12a) When combined with sodium thiosulfate end product is thiocyanate Na2S2O3 + B12 = HSCN + B12a Recycling of hydroxycobalamin Renally cleared Synergistic effect of thiosulfate and B12a Hydroxycobalamin (vitamin B12a) Advantages vs CAK less toxic does not produce MHb (thus appropriate for smoke inhalation victims) may be administered out of hospital cheaper Hydroxycobalamin (vitamin B12a) Available in Europe as Cyanokit 2.5 and 5.0 g doses very concentrated (5g/100 ml) in USA hydroxycobalamin available in 1mg/mL (5L infusion required for 5g dose) No pharmaceutical company willing to sponsor FDA approval and development in North America Cyanide Poisoning - Disposition Symptomatic ICU admision until complete resolution of metabolic acidosis Inhalation exposure Discharge if asymptomatic in ED Cyanide Salt Ingestion Discharge if asymptomatic at 4 hours Cyanogenic glycosides / organonitriles 24 hours of inpatient observation for symptoms Suicidal patients Psychiatric evaluation Methemoglobinemia What is methemoglobinemia? Oxidation of iron within heme from Fe2+ to Fe 3+ •Methemoglobinemia is due to an imbalance of MHb production and MHb reduction MHb - Biochemistry Hemoglobin tetrameric molecule 8 different dimers of MHb are produced when exposed to oxidative stress Oxidized (Fe3+) heme cannot carry oxygen Allosteric changes cause non-oxidized heme to bind oxygen more tightly Left shift of oxygen dissociation curve Thus 30% methemoglobinemia has <70% of original oxygen carrying capacity •Leftward shift of Hb-Oxygen dissociation curve •Impaired oxygen delivery to tissues Biochemistry, continued … Positively charged MHb has high affinity for negative anions (cyanide, fluoride, chloride) Neutral Hb has high affinity for neutral ligands (CO, O2. CO2) ….thus MHb is not particularly good at transporting oxygen (functional anemia) Methemoglobinemia - etiology Spontaneous Congenital Transient (illness associated) Toxic Iatrogenic Spontaneous Methemoglobinemia Autooxidation of Hb 0.5 - 3% Hb converted to MHb each day Autoreduction of MHb 99% occurs via NADH-dependent cytochrome b5 reductase (b5r) pathway Ascorbic acid, glutathione – minor role in reduction Conversion of MHb to Hb is 15% per hour (assuming no ongoing production) A. The NADH-dependent cytochrome b5 methemoglobin reductase system (endogenous). B, The NADPH-dependent methemoglobin reductase system (therapeutic). Source: Ford: Clinical Toxicology Congenital Methemoglobinemia Hemoglobin M rare autosomal dominant disorder stabilize heme iron in ferric (3+) state death in homozygotes lifelong cyanosis in heterozygotes Congenital Methemoglobinemia cytochrome b5 reductase deficiency autosomal recessive lifelong cyanosis in homozygotes …but very few symptoms due to other adaptations very sensitive to xenobiotic oxidizing agents cytochrome b5 deficiency very rare autosomal recessive Congenital Methemoglobinemia NADPH-MHb reductase deficiency exceedingly rare Does not cause MHb Enzyme only reduces MHb in presence of exogenous catalyzing agent (ie: methylene blue) Patient would not respond to therapeutic methylene blue The Fugates of Troublesome Creek Fugate pedigree with genotypes •Congenital NADH-diaphorase deficiency Transient (illness-associated) Methemoglobinemia MHb common in septic infants with gastroenteritis and acidosis Infants <6 months NADH-dependent reductase deficiency Presence of fetal Hb Thus infant Hb more prone to oxidative stress Exact mechanism poorly understood altered flora, RTA, low Cl, UTI, protein intolerance …. Toxic Methemoglobinemia side effect of therapeutic drugs environmental nitrates in well water nitrates in spinach, carrots, beets, etc. intentional OD Toxic Methemoglobinemia Factors influencing degree of MHb 1) 2) 3) 4) rate of entry of oxidant into circulation and RBCs rate of metabolism of toxin in body rate of excretion of toxin effectiveness of cellular MHb reduction systems Toxins causing MHb chloroquine dapsone local anaesthetics methylene blue metoclopramide nitrates nitrites NTG nitroprusside phenacetin pyridium primaquine rifampin sulfonamides vitamin K3 chlorhexidine Therapeutic Methemoglobinemia Iatrogenic induction of MHb in cyanide poisoning Methemoglobinemia - Diagnosis Physical Exam cyanotic “Chocolate brown” lips Symptoms vs MHb concentration MHb conc. %MHb Symptoms <1.5 g/dL <10 None 1.5-3.0 g/dL 10-20 Cyanotic skin 3.0-4.5 g/dL 20-30 Anxiety, lightheadedness, headache, tachycardia 4.5-7.5 g/dL 30-50 Fatigue, confusion, dizziness, tachypnea, tachycardia Coma, seizures, arrhythmias, acidosis 7.5-10.5 g/dL 50-70 >10.5 g/dL >70 death Chocolate-brown arterial blood does not become red with exposure to oxygen filter paper test place drop of blood on filter paper - MHb will not turn red Potassium cyanide test MHb turns red when CN added, sulfhemoglobin does not ABG Measured - pH, pCO2, PO2 Remember … PO2 refers to dissolved oxygen and has nothing to do with Hb Calculated SaO2 – from normal Hb-oxy dissociation curve Assumes all Hb is normal Abnormal Hb (MHb) which do not interfere with pulmonary diffusion with falsely elevate SaO2 “Saturation gap” = measured – calculated sats >5% discrepancy suggests MHb, carboxyhemoglobin, or sulfhemoglobin HCO3 – from Henderson-Hasselbach equation Pulse oximetry Not accurate in MHb!! Only measures 2 wavelengths: 660 & 940nm 100% MHb will read 85% saturation Co-oximetry Measures four wavelengths Maximal absorption peak at 630-631 nm (little interference from oxyhemoglobin) MHb - Treatment Mild cases (no overt hypoxia) Supportive care Remove offending agent (half-life of local anaesthetic induced MHb in normal individual = 55 minutes) Severe Cases overt hypoxia, CNS depression, CVS instability manage more aggressively in patients with coexisting medical problems (CAD, etc.) Recommend antidote for MHb > 30% (or 20% in symptomatic patients) 100% oxygen GI/skin decontamination (charcoal, etc.) Methylene Blue Specific antidote for MHb 1-2 mg/kg over 5 minutes Repeat doses to maximum 7mg/kg A. The NADH-dependent cytochrome b5 methemoglobin reductase system (endogenous). B, The NADPH-dependent methemoglobin reductase system (therapeutic). Source: Ford: Clinical Toxicology Methylene Blue G6PD deficiency – Contraindication Enzyme used in formation of NADPH Insufficient NADPH produced to reduce methylene blue (oxidizing agent) to leukomethylene blue (reducing agent) Relative buildup of methylene blue (oxidizing agent) Can get paradoxical methemoglobinemia and methylene blue induced hemolysis Ascorbic Acid 300-1000mg/day iv (divided tid-qid) Nonenzymatic MHb reduction N-acetylcysteine Works in vitro, no in vivo studies yet Treatment Congenital MHb Generally asymtomatic due to compensatory mechanisms Methylene blue – 100-300mg/day Ascorbic acid – 200-500mg/day Illness associated MHb in infants Supportive care (hydration, etc.) Treat MHb >30% Clinical decision making in methemoglobinemia