File
advertisement
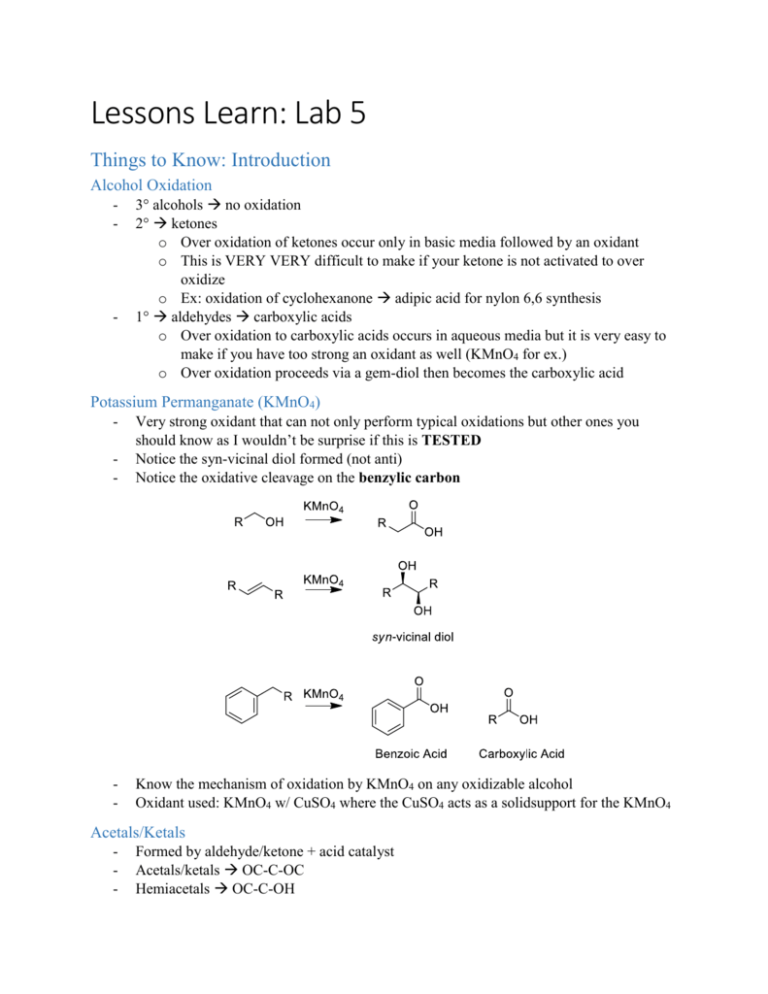
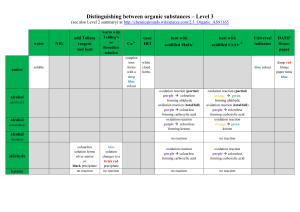
Lessons Learn: Lab 5 Things to Know: Introduction Alcohol Oxidation - - 3° alcohols no oxidation 2° ketones o Over oxidation of ketones occur only in basic media followed by an oxidant o This is VERY VERY difficult to make if your ketone is not activated to over oxidize o Ex: oxidation of cyclohexanone adipic acid for nylon 6,6 synthesis 1° aldehydes carboxylic acids o Over oxidation to carboxylic acids occurs in aqueous media but it is very easy to make if you have too strong an oxidant as well (KMnO4 for ex.) o Over oxidation proceeds via a gem-diol then becomes the carboxylic acid Potassium Permanganate (KMnO4) - Very strong oxidant that can not only perform typical oxidations but other ones you should know as I wouldn’t be surprise if this is TESTED Notice the syn-vicinal diol formed (not anti) Notice the oxidative cleavage on the benzylic carbon - Know the mechanism of oxidation by KMnO4 on any oxidizable alcohol Oxidant used: KMnO4 w/ CuSO4 where the CuSO4 acts as a solidsupport for the KMnO4 Acetals/Ketals - Formed by aldehyde/ketone + acid catalyst Acetals/ketals OC-C-OC Hemiacetals OC-C-OH Benedict’s Test - Need to know what is in it, and how it works (find in introduction) Most importantly know when it gives a positive signal (and what this positive signal looks like) and what it means when you get a positive signal (you have an aldehyde) You should be able to write the reaction transformations that take place in a benedict’s test (ie. Draw an aldehyde that changes to the carboxylic acid) Things to Know: Experiment - - - This experiment is a typical synthesis experiment which means: Know what to expect on a TLC (just like what you saw in the previous test) Be able to draw a flow chart for the experiment if asked o This includes knowing what will be in the aq. phase and what will be in the org phase. Know the conditions of the reactions and be able to troubleshoot if something was to go wrong o Ex: Can’t form acetal? Maybe you didn’t add an acid catalyst o Ex: See more products than expected? Maybe over oxidation? Over oxidation not possible? Maybe oxidation of an impurity or other functional group? Be able to identify 2 unknowns based on the benedicts test