David R. Schoneker - IPEC
advertisement
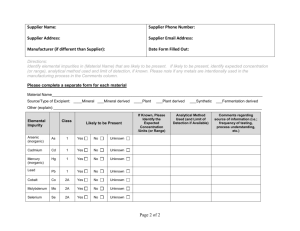
Elemental Impurities (USP, PhEur & ICH Q3D) Excipient Realities and Challenges David R. Schoneker Director of Global Regulatory Affairs dschoneker@colorcon.com KNOWN What do we know today about the levels of metal impurities in excipients? 3 Kaolin & Talc mines Kaolin mine near Kaznejov, Czech Republic Known – at least 12 to 55 ppm Lead from periodic testing Talc extraction in Trimouns Talc Mine, Midi-Pyrenees, France Known – Current USP Spec <10 ppm Lead – only tested once per year www.ipecamericas.org Current Compendia Methodologies • Semi quantitative, non-specific for individual metals (use Pb limit test) • Compendial methods & metals not harmonized USP <231> EP 2.4.8 JP 1.07 • 3 methods described • 8 methods described. • 4 methods described. • Uses Pb ref. std + CH3C(S)NH2-glycerinebase TS • Use of Pb ref. std + CH3C(S)NH2 or Na2S Reagent • Use of Pb ref. std. + Na2S Reagent • Permissible limit expressed in ppm for Pb • Permissible limit expressed in ppm for Pb www.ipecamericas.org • Permissible limit expressed in ppm for Pb Trends and Implementation plans Global desire to control metals with known toxicological concerns in finished drug products Today, there are no known metal impurity issues impacting patient safety HOWEVER, to enhance future monitoring, − ICH is developing Q3D Metal Impurity Guideline, − USP proposed General Chapters <232> Elemental Impurities - Procedures and <233> Elemental Impurities – Methods − PhEur is implementing 2.4.20 Determination of Metal Catalyst or Metal Reagent Residue and 5.20 Metal Catalyst or Metal Reagent Residues Regulators and industry need to establish a consistent/reliable set of expectations to ensure a “right first time” approach to implementation Regulators and industry need to carefully plan and phase-in implementation timelines and approaches for these new requirements in order to allow adequate preparation by suppliers and drug manufacturers and prevent disruption of the drug supply. www.ipecamericas.org Elemental Impurities Coalition for the Rational Implementation of the USP Elemental Impurities Requirements Coalition for the Rational Implementation of the USP Elemental Impurities Requirements Industry Coalition Meetings & Activities Other trade associations and many IPEC-Americas member companies in the United States and Europe ICH Q3D Guideline Scope criteria and limits − Applies to new drug products Excipients outside the direct scope of the guideline Does not include metals intentionally part of the drug product Approach similar to EU Metal Catalysts & Reagents; however, EU focuses on metals utilized in the manufacturing process (catalyst) whereas USP and ICH have extended well beyond metals utilized in the manufacturing process. Limits based on published safety & toxicology data Compendia responsible for methodology, as appropriate ICH advanced the proposed guideline to Step 2 in June 2013 Guideline received through IPEC-Americas on June 20th 2013 Step 2b Guideline published on ICH website on August 5, 2013 Step 2b Guideline will be published in Federal Register in next few weeks Public comment period will occur before the guideline goes to Step 4 www.ipecamericas.org ICH Q3D Step 2 Guideline Incorporates Risk Assessment, Risk Management and Risk Mitigation concepts Testing is not the default; however, where necessary − − − Methods are outside the scope of ICH Q3D Appropriate, validated analytical methods should be used Tests should be specific for each metal (e.g. ICP-AES/ICP-MS) Assessments for each metal of interest based on − − Permitted daily exposure (PDE) for each metal of toxicological concern Permitted use of defined metal impurities concentrations when daily “drug product” dose is NMT (not more than) 10 g/day www.ipecamericas.org Table A.2.1: Permitted Daily Exposures for Elemental impurities1 1PDEs reported in this table are rounded to a 2 significant figures (µg/day). Classification as defined in Section 4 3 Insufficient data to establish an appropriate PDE; the PDE was established based on platinum PDE. 2 Element As Cd Hg Pb Co Mo Se V Ag Au Ir3 Os3 Pd Pt Rh3 Ru3 Tl Ba Cr Cu Li Ni Sb Sn Class2 1 1 1 1 2A 2A 2A 2A 2B 2B 2B 2B 2B 2B 2B 2B 2B 3 3 3 3 2 3 3 Oral PDE μg/day Parenteral PDE, μg/day Inhalation PDE, μg/day 15 5.0 40 5.0 50 180 170 120 170 130 1000 1000 100 1000 1000 1000 8.0 13000 11000 1300 780 600 1200 6400 15 6.0 4.0 5.0 5.0 180 85 12 35 130 10 10 10 10 10 10 8.0 1300 1100 130 390 60 600 640 1.9 3.4 1.2 5.0 2.9 7.6 140 1.2 6.9 1.3 1.4 1.4 1.0 1.4 1.4 1.4 69 340 2.9 13 25 6.0 22 64 USP <232> and <233> USP was unilaterally implementing two elemental impurity general chapters which became official on 1 Feb 2013 − Elemental Impurities - Limits <232> 15 elements defined, some with different limits than ICH Q3D − Elemental Impurities - Procedures <233> Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS) Other methods shown to be “equivalent” Conformance with <232> and <233> in applicable monographs was proposed for May 1, 2014 implementation Based on comments received from the Coalition and other industry groups USP has decided to delay implementation and harmonize limits and implementation timeline with ICH Q3D – BIG INDUSTRY SUCCESS!! - MORE LATER!! www.ipecamericas.org PhEur 2.4.20 & 5.20 The European Pharmacopoeia Commission adopted general chapter − Metal catalysts or metal reagents residues (5.20) − Only need to test/control when used in the mfg. process method − Determination of metal catalysts or metal reagent residues (2.4.20). General approach for determination of metal catalysts or metal reagent residues in substances for pharmaceutical use, to be applied wherever possible. Method and measurement techniques noted include: Atomic Emission Spectroscopy (AES) Atomic Absorption Spectroscopy (AAS) X-ray Fluorescence spectroscopy (XRFS) Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS) Official for pharmaceutical ingredients in September 2013 - ??? 13 EU PhEur 2.4.20/5.20 or ICH Q3D? • PhEur has stated that it will harmonize with ICH Q3D once it moves to Step 4 • Will publish it word for word in PhEur without change (similar to Q3C - Residual Solvents) • However, what will happen in September 2013 with implementation of 2.4.20 and 5.20? • EMA decided to delay the requirements and harmonize existing drug requirements with ICH Q3D on July 24, 2013 • New drugs – still a question??? User Realities What does this all mean to Pharma Companies? PhEur 2.4.20 & 5.20? USP <232> & <233>? ICH Q3D ? Excipient Realities? Confusion ? www.ipecamericas.org Realities for Implementation Requirements will be on the dosage form, NOT the excipients. Excipients do not have to “comply” to Q3D! Many excipients contain metal impurities at the 1 to 10 ppm level. Many users may try to set specifications on excipients; HOWEVER, many suppliers may NOT be willing or able to agree to excipient specifications below those currently required to comply with monographs or other regulatory requirements. Pharmaceutical companies may find it necessary to perform significant elemental testing of excipients and/or drug products in order to determine actual drug product impurity levels and whether they comply with final PDE limits www.ipecamericas.org Realities for Implementation For many excipient companies their pharmaceutical business is a minor part of their sales. Pressures for these companies to perform routine testing or reduce specification limits could lead to: − Cease supporting product sales to pharmaceutical industry − Increased pricing to cover additional costs needed to address new requirements. Some drug products may require changes in excipient sourcing, and perhaps even reformulation in order to meet PDE requirements. Collective implementation efforts will require time to do properly. www.ipecamericas.org Validated Metal Impurity Realities Today little data exists to perform a scientific risk assessment of metal content Unknown Unknowns − Most excipients have not been routinely tested; therefore, current metal concentrations and variability is often UNKNOWN! − Communication between pharma industry and suppliers is critical to best understand what is known and NOT known about metal impurity levels in excipients and APIs Lack of industry data required to confirm actual vs theoretical metal impurity levels − It will take a long time to develop enough data to understand normal variation needed for a science-based risk assessment www.ipecamericas.org Validated Excipient Sources Metal content often inherent (due to sourcing) and cannot be “easily” reduced or removed Plant-derived Excipients − Grown in soil (e.g. cellulose derivatives) − Harvested from the ocean (e.g. alginates, carageenan) Synthetic Excipients − Derived from oil through synthetic processes – may use metal catalysts (e.g. povidone, PEG, silicones) Mineral-based Excipients − Conversion of ores from mines (e.g. TiO2) www.ipecamericas.org Validated Potential Normal Variation Many metal impurities are naturally present (e.g. lead) in mined excipients and cannot be further processed out; therefore, it is important to understand the actual levels present Normal variation can be expected from excursions that occur in the raw material source www.ipecamericas.org Knowns – Limited Industry data Metal impurity examples for mineral based excipients - Supplier COAs Total Inorganic Pb ppm Hg ppm 0.2 max 10.0 max 3.0 max 1.0 max Supplier C 3 max CALCIUM CARBONATE USP PRECIPITATED Supplier D TALC USP/FCC/EP/JP - Item Description Supplier/ Manufacturer As ppm Ca ppm Cr ppm Cu ppm Ni ppm TITANIUM DIOXIDE, USP/FCC/EP/JP Supplier A 1.0 max 1.0 max 1.9 max 0.5 max N/A TITANIUM DIOXIDE USP/EP/JP Supplier B 10.0 max 1.0 max N/A N/A N/A CALCIUM CARBONATE EP 1 max 10 max N/A N/A N/A N/A N/A N/A 3 max 0.5 max N/A N/A N/A Supplier E 3 max N/A 10 max N/A N/A N/A N/A TALC MICRONIZED USP/EP Supplier F 4 max N/A 10 max N/A N/A N/A N/A TALC USP/EP Supplier G 3 max N/A 5 max N/A N/A N/A N/A Unknowns: The following additional metal impurities were not specifications or reported on the Suppliers COAs: Mn, Mo, Pd, Pt, V, Os, Rh, Ru, Ir Coalition 2012 -2013 Activities Called for Data for submission to ICH Q3D EWG - developed blinded sample study with FDA to gain some limited understanding Designed “Information Exchange Request” (to gather data) Published Pharmaceutical Technology article on Elemental/Metal Impurities Filed Formal Appeal with USP Requesting Withdrawal of Elemental Impurities General Chapters and General Notices Established initial FDA meeting (Nov. 20, 2012) to present concerns with USP non-harmonized approach and timeline Developed Tools to help support elemental impurity assessment Set up several Project Teams to work on collecting data to justify the use of acid leach methodology and to assess the technical challenges in performing appropriate ICP-MS methodologies for EI testing www.ipecamericas.org Information Exchange Request Developed standardized request letter and form templates to help facilitate industry communication between users and makers of APIs and excipients. Template created and designed to help pharmaceutical companies: gather information from suppliers pertaining to potential metals/concentrations in both APIs and excipients used in the production of drug products. use information from suppliers to determine potential presence / concentration of each metal in the assessment of a finished drug product Permitted Daily Exposure (PDE) level. NOTE: both API and excipient manufacturers are encouraged to utilize the Information Exchange request template form to proactively develop their own product documentation/information. www.ipecamericas.org Information Exchange Request URGENT industry need for BASE-LINE DATA! IDEAL WORLD…. Pro-actively completed by suppliers and sent to users REAL WORLD… Few suppliers have data or will complete and return the form to users Templates and request letter: http://ipecamericas.org/content/ichq3d-information-exchange-request Formal Appeal/Comments to USP Oct 19, 2012 Feb 1, 2013 Submitted formal appeal to USP requesting withdrawal of April 2012 draft Chapter <232> & <233> USP published <232> and <233> and retained May 1, 2014 implementation date Appeal Denied 1/25/2013 Nov 15, 2012 March 27, 2013 USP postponed publication of <232> and <233> pending review of industry appeals Comments sent to USP concerning the May 2014 planned implementation timeline http://www.usp.org/usp-nf/official-text/revision-bulletins/elemental-impurities-limits-andelemental-impurities-methods www.ipecamericas.org USP Advisory Committees • USP formed an Advisory Committee with two project teams to assess next steps for harmonization and implementation with ICH Q3D – Experts chosen from Coalition members, FDA and others – Project teams have met twice and provided feedback to USP – USP will harmonize <232> limits with ICH Q3D and republish in 2014 (timing not yet established) – Implementation timing TBD – Three options under consideration – decision by October Elemental Impurity Assessment Tools Implementation Timeline Model PDE calculator for component metal information − Sample formulations for dosage forms Examples of industry data comparing total metals vs extractable metals. www.ipecamericas.org Total Metal vs Acid Leached Metal As, Pb and Cd in Smectite Clay Excipients As, ppm Pb, ppm Cd, ppm HCl Leach* <233> Digestion** HCl Leach <233> Digestion HCl Leach <233> Digestion Purified Bentonite NF 0.4 0.7 6.9 12.9 0.03 0.08 MAS NF Type 1A 0.3 1.5 5.5 10.8 0.02 0.09 MAS NF Type 1B <0.1 1.4 3.8 9.2 0.01 0.08 MAS NF Type 1C 0.3 2.1 4.4 10.9 0.02 0.08 MAS NF Type 2A 1.0 1.6 8.6 15.3 0.02 0.08 * Dilute HCl extraction based on the Pb test in the MAS NF monograph; ICP-MS ** Closed vessel microwave digestion with HNO3; ICP-MS Pb analyses using Acid leach tests vs direct biorelevant simulated gastric fluid extraction Magnesium Aluminum Silicate NF Type 2A (Smectite Clay) Pb, ppm Simulated Gastric Fluid Extraction, 2hr (SGF extraction + ICP-MS) 5.9 Monograph specification test (dilute HCl extraction + AA) 9.5 HCl Leach (dilute HCl extraction + ICP-MS) 8.6 “Total” Pb content (closed vessel microwave digestion with HNO3 + ICP-MS) 15.3 Coalition Project Teams • One team is gathering data and drafting a white paper concerning the need to allow for bioaccessability related test methods (acid leach) rather than just total dissolution – To be submitted to ICH Q3D EWG • New team recently started to assess the technical methodology challenges with the use of ICP-MS methods for EI of pharmaceutical materials – possible USP PF Stimuli article and General Chapter – looking for interested parties! – Testing is proving to be much more complex than previously thought FDA Guidance FDA has developed a Guidance pertaining to ICH Q3D and USP 232/233 implementation for Drug Products Marketed in the US. Timeline for publication not known but expected soon Coalition members developed a list of questions (FAQs) for the FDA to consider during guidance development and possibly use to post a Q&A document in the future Preliminary draft FAQs submitted to FDA before USP deferment – Revision was needed since USP has delayed implementation and since ICH Q3D Step 2 guideline published FDA has informed the USP Advisory Committee project team that they used the Coalition FAQs to help them revise their draft guidance document after the USP deferment – they claim to have addressed many of the concerns – WE WILL SEE!! ACTIONS Current Industry Needs • Complete Information exchange requests/provide available information – Suppliers complete initial assessment for each excipient – Users compile information from forms to determine next steps… to be used, as available for PDE calculator and Risk Assessment • • • • • • Establish / validate equipment (ICP-AES/ICP-MS) or identify external test facility Develop “sample preparation” and “analysis” methodologies Train personnel Develop base-line elemental impurity levels for each ingredient used in a drug product – where necessary, monitor for excursions Collaboratively work together (drug manufacturers / ingredient suppliers) to develop (via risk assessment) future testing / reporting strategy for each ingredient used in a drug product. Drug manufacturers use PDE calculator and risk assessments to assure compliance of elemental impurity levels in finished drug product formulations 32 Your Questions/Concerns The information contained in this presentation is proprietary to Colorcon and may not be used or disseminated inappropriately.