Powerpoint - Brain & Cognitive Sciences
advertisement

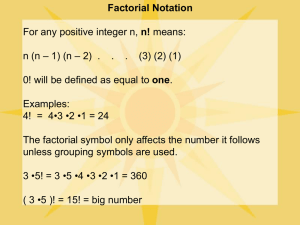
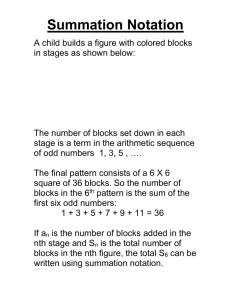
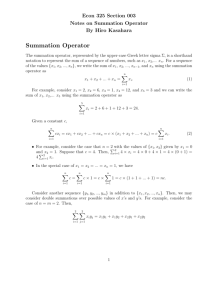
Age-Related Decrements in the Integrative Action of the Auditory Nervous System* seen in the Acoustic Startle Reflex of the CBA Mouse ARO 2003 #198 James R. Ison, Paul D. Allen, Jordan Bell, Catherine A. Moore, Carolyn M. Tyler Brain & Cognitive Sciences, University of Rochester, Rochester, NY RESULTS, Experiment 2 THE EXPERIMENT RESULTS, Experiment 1 a) Acoustic Behavior - maximum summation with 2 ms gap for 1 ms tone pips in rats, then decay: Marsh et al. (1973) J. Comp. Physiol. Psychol. 82:507-511. Subjects: 35 CBA/6J mice were tested, at 6 weeks (n = 7: 4M, 3F); 7 months (n = 12: 5M, 7F); and 24 months of age (n = 16: 10 M, 6F). The mice were maintained with ad libitum food and water in group cages. All procedures were approved as following NIH Guidelines. Seven month old mice responded most vigorously overall to 120 dB tone pips. Very old mice responded vigorously to single pips but showed less summation to double pips than younger mice. Relative ASR shows that the older mice had less temporal integration and less peak summation but the longest summation window. (Mean & SEM are shown.) 600 120 dB b) CN electrical stimulation – maximum summation for .1 ms shocks at 1-3 ms separation, then decay: Yeomans et al. (1989) Brain Res. 486:147-158. Apparatus and procedure: A photograph of the mouse in situ - ASR (av-Units) c) Explanation - Initial increased summation indicates "decaying refractoriness" from S1 activation while later the decline from peak summation indicates "decaying excitation" adding to S2. 400 200 Mice received 110 (7 Mo) 120 (6 wk) or 130 dB (24 mo) tone pips. The overall ASR levels were more similar here across groups but again relative peak summation was weaker and delayed in the oldest mice, and their summation window was longer. (Mean & SEM are shown.) 6 wks. 120 dB 7 mo 110 dB 300 24 mo 130 dB ASR (av-Units) RESEARCH ON 2-PULSE SUMMATION IN ASR 200 100 PLAUSIBLE BASIS IN MEMBRANE BIOPHYSICS Relative Response C 0 1 2 3 4 5 6 b) The later decline in summation results as low-threshold K+ channels (such as Kv1) rectify baseline potential following an EPSP, so that an S1 EPSP does not summate with an S2 EPSP. IMPLICATIONS FOR AGE EFFECTS ON SUMMATION 5 4 6 wks. 7 mo. 24 mo. 3 2 1 0 C 0 1 2 3 4 5 6 10 Stimulus Onset Asynchrony (ms) Old mice show less expression of both Kv3.1 and Kv1.1, thus** The small test cage is mounted on an acrylic platform to (a) following an AP, cell potentials should more slowly return to baseline in old mice, retarding the growth of summation, which an accelerometer is attached. The force of the reflexive (**Inferred from Brew & Forsythe, 1995, J Neurosci, 15:18111822) 10 flinch to the overhead tone bursts (from TDT equipment) is integrated for 100 ms following tone onset. 14 stimulus At "C" a single pip is 120 dB; at "0" a single pip is 123 dB; at "1" the 120 dB pip is 2 ms long. All groups show temporal integration and summation, but old mice have more "jitter." conditions were given on average 20 s apart, 11 trials per condition in a semirandom order, with single tone pips, or double pips separated by 0 to 9 ms gaps. Work supported by NIA, AG09524, by the Schmitt Program on Integrative Brain Research, and by NIDCD (CNCS) P30DC05409. The idea for this work grew out of conversations with Helen Brew, Josh Gittelman, and Bruce Tempel in Seattle WA, April 2002 . * With apologies to C.S. Sherrington Relative Response a) Initial increased summation results as high-threshold K+ channels (such as Kv3) rectify baseline potential following the S1 AP, and thus permit a second response to S2 (b) but when an EPSP is present without an AP, then these nerve fibers should more slowly return to baseline, this resulting in an overall longer summation time. 0 0 C 0 1 2 3 4 5 6 10 C 0 1 2 3 4 5 6 10 3 2 1 0 Stimulus Onset Asynchrony (ms) CONCLUSIONS Old mice respond as vigorously as younger mice for brief stimuli but show reduced peak summation for double pulses, while their summation window is longer. These data are consistent with the understanding that old mice express fewer fast acting K+ channels that return membrane potentials to baseline following a perturbation. This loss must diminish the temporal precision of neural processing, which may be expected to degrade sensory function in many auditory tasks.