Finding Heat Changes
advertisement

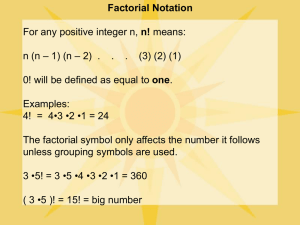
Computer Applications in Chemical Engineering Using MathCad to do Integration, Summation, etc. In Chemical Engineering it is frequently necessary to find the area under a curve (compute an integral) either numerically or analytically. MathCad makes this easy by having both symbolic and numerical integration and summation functions. The Thermodynamic definition of the heat capacity is: 𝑐ℎ𝑎𝑛𝑔𝑒 𝑖𝑛 𝑄 𝑑𝑄 = = 𝐶𝑝 𝑐ℎ𝑎𝑛𝑔𝑒 𝑖𝑛 𝑇 𝑑𝑇 When integrated (the area under the curve is found), this equation gives the amount of heat “Q” required to raise the temperature of a unit mass of a pure substance from one temperature to another, assuming no phase change. The heat capacity of liquid water is reported to be: Cp(T)=a+bT+cT2+dT3 (kJ/mole-K) where a=33.46103, b=0.6880×10-5, c=0.7604×10-8, d=-3.593×10-12. 1. 2. 3. Use symbolic integration to compute the amount of heat required to raise one mole of liquid water from 22 oC to 91 oC. Do the same calculation numerically. Do the same calculation using a summation. Illustrate how your choice of integration step size affects the results as compared to the analytical solution. In thermodynamics, the work done on a system is defined as: 𝑉2 𝑊 = − ∫ 𝑃𝑑𝑉 𝑉1 where, P is the pressure as a function of volume, V is the volume and V1 and V2 are the initial and final volumes in a system. 4. 5. 6. 7. Find the amount of work required to compress one mole of steam (water vapor) at a constant temperature of 600 K from a volume of 100 L to 50 L (L=liters). Do this calculation using the built in numerical integration function using both the “Adaptive” and “Romberg” integration. Use the trapezoidal rule and a summation also to estimate the work. Comment on the effect that integration step size has on accuracy. Attempt to do the integration analytically. Comment if you cannot make this work Explain why your choice of step size has very little affect when you integrate Cp(T), but has more affect when you integrate P(V). HINT: Consider plotting Cp(T) vs T and compare it to P(V) vs V.