Chapter 6 by Miss Anis
advertisement

ERT 210
Process Control & dynamics
CHAPTER 8
Dynamic Response Characteristics of
More Complicated Processes
Miss Anis Atikah binti Ahmad
OUTLINE
1. Poles and Zeros
and Their Effect on
Process Response
6 6
Chapter
Chapter
2. Process with
Time Delays
Dynamic Response
Characteristics
3. Approximation of
Higher-Order
Transfer Functions
4. Interacting and
Noninteracting
Processes
2
5. Multiple-Input,
Multiple-Output
(MIMO) Processes
1. Poles and Zeros and Their Effect on Process Response
• General Representation of standard transfer function form:
- There are two equivalent representations:
6 6
Chapter
Chapter
m
G s
i
b
s
i
i 0
n
i
a
s
i
bm s m bm 1s m 1 ... b0
(4-40)
n
an s an 1s n 1 ... a0
(6-2)
i 0
s zm
G s
an s p1 s p2 s pn
bm s z1 s z2
(6-7)
where {zi} are the “zeros” and {pi} are the “poles”.
3
Poles and Zeros and Their Effect on Process Response
• Consider a particular transfer function;
6 6
Chapter
Chapter
K
, where 0 < 1.
G( s)
2
s( 1s 1)( 2 s 2 2 2 s 1)
The roots of these factors are
s1 0
s2
1
1
1 2
s3
j
2
2
1 2
s4
j
2
2
The values of s
that are the
denominator
polynomial-refer
as poles
(6-1)
Poles and Zeros and Their Effect on Process Response
POLES:
s1 0
s2
1
1
Chapter 6
1 2
s3 j
2
2
1 2
s4 j
2
2
5
Figure 6.1 Poles of G(s) plotted
in the complex s plane.
(X denotes a pole location)
Summary: Effects of Pole and Zero Locations
1. Poles
• Pole in “right half plane (RHP)”: results in unstable system (i.e., unstable
Example
step responses)
Chapter 6 6
Chapter
Imaginary axis
x
x
x = unstable pole
Real axis
(grows with time)
x
• Complex pole: results in oscillatory responses (contains sine and cosine terms)
Imaginary axis
Example
x = complex poles
x
Real axis
x
6
2. Zeros
Note: Zeros have no effect on system stability.
6 6
Chapter
Chapter
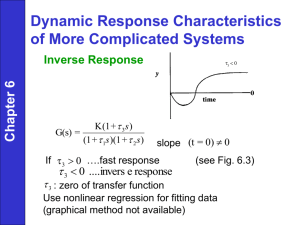
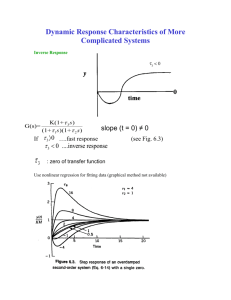
• Zero in RHP: results in an inverse response to a step change in
the input
Imaginary axis
x
Real
axis
y
inverse
response
0
t
• Zero in left half plane: may result in “overshoot” during a step
response (see Fig. 6.3).
7
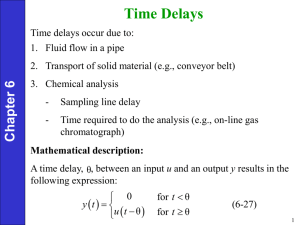
2. Process with Time Delays
Transportation
lag/ transport
delay/ dead time
Chapter 6
θ
Ѳ= Time taken to transport
fluid from point 1 to point 2
Mathematical description:
• A time delay, between an input u and an output y results in the
following expression:
for t θ
0
y t
(6-27)
u t θ for t θ
Transfer Function Representation:
Y s
8
U s
e θs
(6-28)
Chapter 6
2. Process with Time Delays
(cont’)
9
3. Approximation of Higher-Order Transfer
Functions
• Two widely used approximations are:
1. Taylor Series Expansion:
Chapter 6
e
θs
θ 2 s 2 θ3 s 3 θ 4 s 4
1 θs
2!
3!
4!
(6-34)
The approximation is obtained by truncating after only a few
terms.
2. Padé Approximations:
Many are available. For example, the 1/1 approximation is,
e θs
10
θ
1 s
2
θ
1 s
2
(6-35)
3. Approximation of Higher-Order Transfer Functions
(cont’)
• The 2/2 Padé Approximations approximation;
Chapter 6
e θs
θs θ 2 s 2
1
2
12
G2 ( s )
θs θ 2 s 2
1
2
12
(6.37)
Note:
• Please refer page 138 and 139 for more explanations.
11
Example 6.5
Chapter 6
The trickle-bed catalytic reactor shown in Fig. 6.8 utilizes product
recycle to obtain satisfactory operating conditions for temperature
and conversion. Use of a high recycle rate eliminates the need for
mechanical agitation. Concentrations of the single reactant and the
product are measured at a point in the recycle line where the
product stream is removed. A liquid phase first-order reaction is
involved.
Under normal operating conditions, the following assumption may
be made:
1.
2.
3.
4.
12
The reactor operates isothermally so that the reaction rate k is
constant.
All flow rates and the liquid volume V are constant.
No reaction occurs in the piping. The dynamics of the exit and recycle
lines can be approximated as constant time delay θ1 and θ2, as
indicated in the figure. Let c1 denote the reactant concentration at the
measured point.
Because of the high recycle flow rate, mixing in the reactor is
complete.
Example 6.5 (cont’)
6 6
Chapter
Chapter
Question:
'
'
C
(
s
)
/
C
i ( s ).
1. Derive an expression for the transfer function 1
2. Using the following information, calculate ci' (t ) for a step change
'
3
in ci (t ) 2000 kg/m .
Parameter Values:
V = 5 m3
q = 0.05 m3/min
k = 0.04 min-1
13
α = 12
θ1 = 0.9 min
θ2 = 1.1 min
Figure 6.8: Schematic diagram of a trickle-bed reactor with recycle line.
(AT: analyzer transmitter; θ1: time delay associated with material flow
from reactor outlet to the composition analyzer; θ2: time delay associated
with material flow from transmitter to reactor inlet.
6 6
Chapter
Chapter
Solution
(a) Equation 2.66 is applicable only to an isothermal stirred-tank reactor
without recycle. Hence, we make component balance around the
reactor.
(6.39)
dc
V
qci qc2 (1 )qc Vkc
dt
where the concentration of the reactant is denoted by c. Equation 6.39
is linear with constant coefficients. Subtracting the steady-state
equation and substituting deviation variables yields
dc '
V
qci 'qc2 '(1 ) qc 'Vkc'
dt
(6.40)
Additional relations are needed for c2'(t) and c1'(t). They can be
obtained from assumption (iii), which states that the exit and recycle
lines can be modeled as time delays:
(6.41)
c ' (t ) c' (t )
1
14
1
c2 ' (t ) c1 ' (t 2 )
(6.42)
Equations 6-40 through 6.42 provide the process model for the
isothermal reactor with recycle. Taking the Laplace transform of each
equation yields
sVC ' ( s ) qCi ' ( s ) qC2 ' ( s ) (1 )qC ' ( s ) VkC' ( s )
6 6
Chapter
Chapter
C1 ' ( s ) e 1s C ' ( s )
(6.43)
(6.44)
C2 ' ( s ) e 2 s C1 ' ( s )
e (1 2 ) s C ' ( s )
e 3 s C ' ( s )
(6.45)
where θ3 = θ1+ θ2. Substitute (6.45) into (6.43) and solve for the output
C'(s):
C ' (s)
15
sV qe 3 s
q
Ci ' ( s )
(1 )q Vk
(6.46)
Equation 6.46 can be rearranged to the following form:
C ' ( s)
K
Ci ' ( s )
3 s
s 1 K (1 e )
(6.47)
where
q
q Vk
V
q Vk
(6.48)
6 6
Chapter
Chapter
K
(6.49)
Note that, in the limit as 3 0, e s 1 and
3
C ' ( s)
K
Ci ' ( s )
s 1
(6.50)
hence K and can be interpreted as the process gain and time constant,
respectively, of a recycle reactor with no time delay in the recycle line,
which is equivalent to a stirred isothermal reactor with no recycle.
16
The desired transfer function C1'(s)/Ci' (s) is obtained by combining
Eqs. 6-47 and 6-44 to obtain
6 6
Chapter
Chapter
C1 ' ( s )
Ke 1s
Ci ' ( s ) s 1 K (1 e 3 s )
(6.51)
(b) To find c1'(t) when ci'(t) = 2000 kg/m3, we multiply (6.51) by 2000/s
2000 Ke1s
C1 ' ( s )
s[s 1 K (1 e 3s )
(6.52)
and take the Laplace transform. From (6.52), it is clear that the numerator time
delay can be inverted directly; however, there is no transform in Table 3.1 that
contains a time-delay term in the denominator.
• To obtain an analytical solution, the denominator time delay term must be
eliminated by introducing a rational approximation, for example, the 1/1 Padé
approximation in (6.35).
G1 ( s ) G1 ( s )
17
1
1
2
2
s
s
(6.35)
Substituting (6.35) and rearranging yields
2000 K 3 s 1e 1s
2
C1 ' ( s )
s 3 s 2 3 K 3 s 1
2
2
(6.53)
6 6
Chapter
Chapter
This expression can be written in the form
2000 K a s 1e 1s
C1 ' ( s )
s ( 1s 1)( 2 s 1)
(6.54)
where a = θ3/2 and 1 and 2 are obtained by factoring the expression in
brackets. For αKθ3 > 0, 1 and 2 will be real and distinct.
The numerical parameters in (6.53) are:
q
0.05
0.2
q Vk
0.05 (5)(0.04)
V
20 min
q Vk
K
18
Substituting the values in (6.53), we obtain
400s 1e0.9 s
400s 1e0.9 s
C1 ' (s)
2
s 20s (20 1 (24)(0.2)(1)) s 1 s(25s 1)(0.8s 1)
(6.55)
Taking inverse Laplace and introducing the delayed unit step function
S(t - 0.9) gives;
Chapter 6
c1 ' (t ) 400(1 0.99174e (t 0.9) / 25 0.00826e (t 0.9) / 0.8 ) S (t 0.9)
(6.56)
19
Figure 6.9 Recycle reactor composition measured at analyzer:
(a) complete response; (b) detailed view of short-term response.
3. Approximation of Higher-Order Transfer
Functions
6 6
Chapter
Chapter
In this section, we present a general approach for approximating
high-order transfer function models with lower-order models that
have similar dynamic and steady-state characteristics.
In Eq. 6-34 we showed that the transfer function for a time delay
can be expressed as a Taylor series expansion. For small values
of s, after the first-order term provides a suitable approximation:
e θ 0 s 1 θ 0 s
e θs
20
θ 2 s 2 θ3 s 3 θ 4 s 4
1 θs
2!
3!
4!
(6-57)
(6-34)
• An alternative first-order approximation consists of the transfer
function,
6 6
Chapter
Chapter
e
θ 0 s
1
e
θ0 s
1
1 θ0 s
(6-58)
where the time constant has a value of θ0 .
• Equations 6-57 and 6-58 were derived to approximate timedelay terms.
• However, these expressions can also be used to approximate
the pole or zero term on the right-hand side of the equation by
the time-delay term on the left side.
21
Skogestad’s “half rule”
• Skogestad (2002) has proposed a related approximation method
for higher-order models that contain multiple time constants.
6 6
Chapter
Chapter
• He approximates the largest neglected time constant in the
following manner.
• One half of its value is added to the existing time delay (if any)
and the other half is added to the smallest retained time
constant.
• Time constants that are smaller than the “largest neglected time
constant” are approximated as time delays using (6-58).
1
e θ 0 s
e
22
θ0 s
1
1 θ0 s
(6-58)
Example 6.4
Consider a transfer function:
6 6
Chapter
Chapter
G s
K 0.1s 1
5s 1 3s 1 0.5s 1
(6-59)
Derive an approximate first-order-plus-time-delay model,
Keθs
G s
τs 1
using two methods:
(6-60)
(a) The Taylor series expansions of Eqs. 6-57 and 6-58.
(b) Skogestad’s half rule
Compare the normalized responses of G(s) and the approximate
23 models for a unit step input.
Solution
(a) The dominant time constant (5) is retained. Applying
the approximations in (6-57) and (6-58) gives:
0.1s 1 e0.1s
(6-61)
Chapter 6
and
1
e3s
3s 1
1
e0.5 s
0.5s 1
(6-62)
Substitution into (6-59) gives the Taylor series approximation GTS s :
Ke 0.1s e3s e0.5 s Ke 3.6 s
GTS s
5s 1
5s 1
24
(6-63)
(b) To use Skogestad’s method, we note that the largest neglected
time constant in (6-59) has a value of three.
6 6
Chapter
Chapter
• According to his “half rule”, half of this value is added to the
next largest time constant to generate a new time constant
τ 5 0.5(3) 6.5.
• The other half provides a new time delay of 0.5(3) = 1.5.
• The approximation of the RHP zero in (6-61) provides an
(6-61)
additional time delay of 0.1. 0.1s 1 e0.1s
• Approximating the smallest time constant of 0.5 in (6-59) by
(6-58) produces an additional time delay of 0.5.
• Thus the total time delay in (6-60) is,
θ 1.5 0.1 0.5 2.1
G s
25
K 0.1s 1
5s 1 3s 1 0.5s 1
(6-59)
and G(s) can be approximated as:
Ke2.1s
GSk s
6.5s 1
(6-64)
6 6
Chapter
Chapter
The normalized step responses for G(s) and the two approximate models
are shown in Fig. 6.10. Skogestad’s method provides better agreement
with the actual response.
Figure 6.10 Comparison
of the actual and
approximate models for
Example 6.4.
26
Example 6.5
Consider the following transfer function:
6 6
Chapter
Chapter
G s
K 1 s e s
12s 1 3s 1 0.2s 1 0.05s 1
(6-65)
Use Skogestad’s method to derive two approximate models:
(a) A first-order-plus-time-delay model in the form of (6-60)
(b) A second-order-plus-time-delay model in the form:
Keθs
G s
τ1s 1 τ2 s 1
(6-66)
Compare the normalized output responses for G(s) and the
approximate models to a unit step input.
27
Solution
(a) For the first-order-plus-time-delay model, the dominant time
constant (12) is retained.
6 6
Chapter
Chapter
• One-half of the largest neglected time constant (3) is allocated to
the retained time constant and one-half to the approximate time
delay.
• Also, the small time constants (0.2 and 0.05) and the zero (1) are
added to the original time delay.
• Thus the model parameters in (6-60) are:
3.0
θ 1
0.2 0.05 1 3.75
2
3.0
τ 12
13.5
2
28
6 6
Chapter
Chapter
(b) An analogous derivation for the second-order-plus-time-delay
model gives:
0.2
θ 1
0.05 1 2.15
2
τ1 12,
τ 2 3 0.1 3.1
In this case, the half rule is applied to the third largest time
constant (0.2). The normalized step responses of the original and
approximate transfer functions are shown in Fig. 6.11.
Figure 6.11 Comparison
of the actual model and
approximate models for
Example 6.5. The actual
and second-order model
responses are almost
indistinguishable.
29
4. Interacting and Noninteracting Processes
• Consider a process with several invariables and several output
variables. The process is said to be interacting if:
Each input affects more than one output.
6 6
Chapter
Chapter
or
A change in one output affects the other outputs.
Otherwise, the process is called noninteracting.
• As an example, we will consider the two liquid-level storage
systems shown in Figs. 4.3 and 6.13.
• In general, transfer functions for interacting processes are more
complicated than those for noninteracting processes.
30
6 6
Chapter
Chapter
Figure 4.3. A noninteracting system:
two surge tanks in series.
31
Figure 6.13. Two tanks in series whose liquid levels interact.
6 6
Chapter
Chapter
Figure 4.3. A noninteracting system:
two surge tanks in series.
dh1
qi q1
dt
Mass Balance:
A1
Valve Relation:
1
q1 h1
R1
(4-48)
(4-49)
Substituting (4-49) into (4-48) eliminates q1:
32
dh1
1
A1
qi h1
dt
R1
(4-50)
6 6
Chapter
Chapter
Putting (4-49) and (4-50) into deviation variable form gives
dh1
1
A1
qi h1
dt
R1
(4-51)
1
q1 h1
R1
(4-52)
The transfer function relating H1 s to Q1i s is found by
transforming (4-51) and rearranging to obtain
H1 s
R1
K1
Qi s A1R1s 1 τ1s 1
(4-53)
where K1 = R1 and τ1 = A1R1. Similarly, the transfer function
relating Q1 s to H1 s is obtained by transforming (4-52).
33
Q1 s 1
1
H1 s R1 K1
(4-54)
6 6
Chapter
Chapter
The same procedure leads to the corresponding transfer functions
for Tank 2,
H 2 s
R2
K2
(4-55)
Q2 s A2 R2 s 1 τ 2 s 1
Q2 s
1
1
H 2 s R2 K 2
(4-56)
where K2 = R2 and τ2 = A2 R2. Note that the desired transfer
function relating the outflow from Tank 2 to the inflow to Tank 1
can be derived by forming the product of (4-53) through (4-56).
34
Q2 s Q2 s H 2 s Q1 s H1 s
Qi s H 2 s Q1 s H1 s Qi s
(4-57)
Q2 s
1 K 2 1 K1
Qi s K 2 τ 2 s 1 K1 τ1s 1
(4-58)
6 6
Chapter
Chapter
or
which can be simplified to yield
Q2 s
1
Qi s τ1s 1 τ 2 s 1
(4-59)
a second-order transfer function (does unity gain make sense on
physical grounds?). Figure 4.4 is a block diagram showing
information flow for this system.
35
Block Diagram for
Noninteracting Surge Tank
System
Figure 4.4. Input-output model for two liquid surge tanks in series.
36
6 6
Chapter
Chapter
Dynamic Model of An Interacting Process
Figure 6.13. Two tanks in series whose liquid levels interact.
1
q1 h1 h2
R1
(6-70)
The transfer functions for the interacting system are:
37
H 2 s
R2
2 2
Qi s τ s 2ζτs 1
(6-74)
6 6
Chapter
Chapter
Q2 s
1
2 2
Qi s τ s 2ζτs 1
H1 s
K1 τ a s 1
2 2
Qi s τ s 2ζτs 1
(6-72)
where
τ1 τ 2 R2 A1
τ= τ1τ 2 , ζ =
, and τ a = R1R2 A2 / R1 R2
2 τ1τ 2
In Exercise 6.15, the reader can show that ζ > 1 by analyzing the
denominator of (6-71); hence, the transfer function is
overdamped, second order, and has a negative zero.
38
Model Comparison
Noninteracting system
Q2 s
1
Qi s τ1s 1 τ 2 s 1
(4-59)
where τ1
= A1 R1 and τ 2 = A2 R2 .
• Interacting system
Q2 s
1
2 2
Qi s τ s 2ζτs 1
where ζ 1 and τ
= τ1τ 2
• General Conclusions
1. The interacting system has a slower response.
(Example: consider the special case where = 1 2.
2. Which two-tank system provides the best damping
of inlet flow disturbances?
39
5. Multiple-Input, Multiple-Output
(MIMO) Processes
6 6
Chapter
Chapter
• Most industrial process control applications involved a
number of input (manipulated) and output (controlled)
variables.
• These applications often are referred to as multiple-input/
multiple-output (MIMO) systems to distinguish them
from the simpler single-input/single-output (SISO)
systems that have been emphasized so far.
• Modeling MIMO processes is no different conceptually
than modeling SISO processes.
40
• For example, consider the system illustrated in Fig. 6.14.
• Here the level h in the stirred tank and the temperature T are to
be controlled by adjusting the flow rates of the hot and cold
streams wh and wc, respectively.
6 6
Chapter
Chapter
• The temperatures of the inlet streams Th and Tc represent
potential disturbance variables.
• Note that the outlet flow rate w is maintained constant and the
liquid properties are assumed to be constant in the following
derivation.
(6-88)
41
6 6
Chapter
Chapter
Figure 6.14. A multi-input, multi-output thermal mixing process.
42
6 6
Chapter
Chapter
Figure 6.15. Block
diagram of the MIMO
thermal mixing system
with variable level.
43
6 6
Chapter
Chapter
THANK YOU
44