Vlietinck - World Agroforestry Centre
advertisement

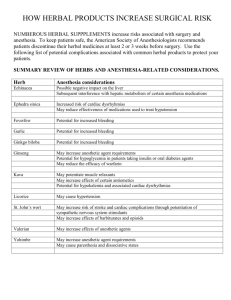
AFRICA HERBAL ANTIMALARIAL MEETING ORGANISED BY THE WORLD AGROFORESTRY CENTRE (ICRAF) AND THE ASSOCIATION FOR THE PROMOTION OF TRADITIONAL MEDICINE (PROMETRA) MARCH, 20th TO 22nd 2006 ICRAF HOUSE NAIROBI, KENYA NEW EUROPEAN LEGISLATION FOR HERBAL MEDICINAL PRODUCTS (HMPs) - NEW OPPORTUNITIES FOR AFRICAN PHARMACEUTICAL INDUSTRIES? PROF. A.J. VLIETINCK UNIVERSITY OF ANTWERP (UA) B-2610 ANTWERP, BELGIUM • CHAIRMAN OF THE GROUP OF EXPERTS 13A OF THE EUROPEAN PHARMACOPOEIA (EP) • MEMBER OF THE COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) OF THE EUROPEAN MEDICINES AGENCY (EMEA) CONTENTS 1. INTRODUCTION 2. LEGAL FRAMEWORK FOR HMPs IN THE EU 2.1. MARKETING AUTHORISATION (MA) PROCEDURES 2.2. MA OF WELL-ESTABLISHED HMPs 2.3. REGISTRATION OF TRADITIONAL HMPs 3. EUROPEAN PHARMACOPOEIA MONOGRAPHS OF HERBAL DRUGS AND HERBAL PREPARATIONS 4. NOTE FOR GUIDANCE ON SPECIFICATIONS: TEST PROCEDURES AND ACCEPTANCE CRITERIA 5. MA OR REGISTRATION OF ANTIMALARIALS FROM ARTEMISIA ANNUA 6. CONCLUSION LEGAL STATUS OF HERBAL PRODUCTS WORLDWIDE FOOD DIETARY SUPPLEMENT DIETARY SUPPLEMENTS DRUG NUTRACEUTICALS HERBAL PRODUCTS HERBAL MEDICINAL PRODUCTS FUNCTIONAL FOODS • • • • • • • DESIGNER FOODS FITNESS FOODS FOODACEUTICALS LONGEVITY FOODS MEDICINAL FOODS NUTRITIONAL FOODS THERAPEUTIC FOODS • • • • • • PHARMACEUTICAL FOODS PHARMACOFOODS PHARMAFOODS PHARMAFOODICALS PRESCRIPTION FOODS . SUPER FOODS HERBAL MEDICINAL PRODUCTS PRESCRIBED BY MEDICAL DOCTORS PRESCRIPTION SHARES BY COUNTRY IN % CURRENT LEGAL FRAMEWORK FOR HMPs IN THE EU DIRECTIVE 2001/83/EC, AS AMENDED (12.06.2003) • ART. 1 : DEFINITION OF MEDICINAL PRODUCTS (a) ANY SUBSTANCE OR COMBINATION OF SUBSTANCES PRESENTED AS HAVING PROPERTIES FOR TREATING OR PREVENTING DISEASE IN HUMAN BEINGS (b) ANY SUBSTANCE OR COMBINATION OF SUBSTANCES WHICH MAY BE USED IN OR ADMINISTERED TO HUMAN BEINGS WITH A VIEW TO MAKING A MEDICAL DIAGNOSIS OR TO RESTORING, CORRECTING OR MODIFYING PHYSIOLOGICAL FUNCTIONS BY EXERTING A PHARMACOLOGICAL, IMMUNOLOGICAL OR METABOLIC ACTION • WHEREBY “SUBSTANCE …. ANY MATTER E.G. VEGETABLE: PLANTS, PARTS OF PLANTS, VEGETABLE SECRETIONS, EXTRACTS …” • ESTABLISHES THAT PROOF OF QUALITY, SAFETY AND EFFICACY IS A PRECONDITION FOR DELIVERING A MARKETING AUTHORIZATION FOR A MEDICINAL PRODUCT. CURRENT LEGAL FRAMEWORK FOR HERBAL MEDICINAL PRODUCTS MA PROCEDURES London Brussels National Mutual recognition Centralised (?) Marketing authorisation : “A medicinal product may only be placed on the market in the European Union when a marketing authorisation has been issued by the competent authority of a Member State for its own territory (national authorisation) or when an authorisation has been granted in accordance with Regulation (EEC) No. 2309/93 for the entire Community (a Community authorisation).” FRIAS, 2004 HMPC – HERBAL COMMITTEE Composition Chair : K. Keller + Vice-Chair : H. Pittner 1 member/MS + 1 alternate/MS + max. 5 co-opted members + accompanied by experts + 5 CO-OPTED MEMBERS 1 HERBAL MEDICINAL PRODUCTS (HMPs) IN THE EU • REGULATORY GUIDANCE ON SOLID GROUNDS REGULATION No 726/2004 (EC) OF 31.03.2004 TITLE IV, THE EUROPEAN MEDICINES AGENCY (EMEA) RESPONSIBILITIES AND ADMINISTRATIVE STRUCTURE ARTICLE 56 1. (d) HMPs • DIRECTIVE 2001/83/EC AS AMENDED BY DIRECTIVE 2004/24.EC AND DIRECTIVE 2004/27/EC OF 31.03.2004 HMPs IN THE EU : ACCESS TO THE MARKET MARKETING AUTHORIZATION (MA) 1. FULL DOCUMENTATION WITH NEW TESTS AND TRIALS 2. FULL BIBLIOGRAPHIC DOCUMENTATION (WELL-ESTABLISHED USE) 3. MIXED APPLICATIONS REGISTRATION 4. SIMPLIFIED DOSSIER FOR TRADITIONAL HERBAL MEDICINAL PRODUCTS CURRENT LEGAL FRAMEWORK FOR HMPs IN THE EU * DIRECTIVE 2003/63/EC (UPDATED ANNEX I TO THE DIR. 2001/83/EC) DOSSIER REQUIREMENTS • SPECIFIC REQUIREMENTS FOR THE DOCUMENTATION AND EXPERT REPORTS ON QUALITY, SAFETY AND EFFICACY • SPECIFIC PART ON APPLICATIONS FOR HMPs • SPECIFIC PART ON BIBLIOGRAPHIC APPLICATIONS • PRESENTATION AND FORMAT OF AN APPLICATION - OLD NOTICE TO APLLICANTS (NTA) 1998 EDITION (PARTS I- IV) - NEW COMMON TECHNICAL DOCUMENT (CTD) 2001 EDITION (MODULES 1 - 5) • TRANSITION FOR “OLD” HMPs APPLICATIONS UNTIL 30.04. 2005 • MANDATORY FOR “NEW” HMPs APPLICATIONS SINCE 01.07.2003 Part I PRESENTATION AND CONTENT OF THE DOSSIER Part II Module 1 Pharmaceutical Regional Information 1.0 Part III CTD Table of Contents 2.1 Toxicological CTD Introduction 2.2 Part IV Quality Overall Summary 2.3 Clinical 1998 EDITION- NTA Non-clinical Overview 2.4 Non-clinical Summaries 2.6 Clinical Overview 2.5 Clinical Summary 2.7 Module 3 Module 4 Module 5 Quality 3.0 Nonclinical Study Reports 4.0 Clinical Study Reports 5.0 FRIAS, 2004 2001 EDITION- CTD 1 LEGAL BASIS FOR SUBMISSION OF HMPs IN THE EU * COMPLETE AND INDEPENDENT APPLICATION / STAND ALONE APPLICATION • ART. 8. 3 (i) OF DIR. 2001/83/EC “COMPLETE DOSSIER APPLICATION” BASED ON THE MANUFACTURER’S OWN QUALITY, PRECLINICAL AND CLINICAL RESEARCH DATA * BIBLIOGRAPHIC APPLICATION • ART. 10.1 (a) (ii) OF DIR. 2001/83/EC 1. IN DEROGATION OF ART. 8.3.(i), AND ….. (a) THE APPLICANT SHALL NOT BE REQUIRED TO PROVIDE THE RESULTS OF PHARMACOLOGICAL AND TOXICOLOGICAL TESTS OR THE RESULTS OF CLINICAL TRIALS IF HE CAN DEMONSTRATE : ii) …. BY DETAILED REFERENCE TO PUBLISHED SCIENTIFIC LITERATURE THAT THE CONSTITUENT OR CONSTITUENTS OF THE PROPRIETARY MEDICINAL PRODUCT HAVE A WELL ESTABLISHED MEDICINAL USE, WITH RECOGNIZED EFFICACY AND AN ACCEPTABLE LEVEL OF SAFETY • CAN BE A “MIXED DOSSIER” APPLICATION INCLUDING BOTH EXISTING BIBLIOGRAPHIC EVIDENCE SUPPLEMENTED WITH THE APPLICANT’S OWN RESEARCH DATA. * ALTHOUGH THE FULL AND BIBLIOGRAPHIC LEGAL BASES HAVE BEEN TRANSPOSED INTO THE NATIONAL LEGISLATION, THERE ARE MAJOR DISCREPANCIES BETWEEN THE MS IN THE CLASSIFICATION AS WELL AS IN THE DOSSIER REQUIREMENTS FOR OBTAINING A MARKETING AUTHORIZATION. “ WELL-ESTABLISHED USE” DEMONSTRATION (WEU) (1) ANNEX 1 TO CD 2001/83 EC AMENDED BY CD 2003/63 (25.06.2003) CTD 3.2 (a) : • THE TIME OVER WHICH A SUBSTANCE HAS BEEN USED, NOT LESS THAN 10 YEARS FROM FIRST AND SYSTEMATIC USE AS A MEDICINAL PRODUCT; • QUANTITATIVE ASPECTS OF THE USE OF THE SUBSTANCE; • THE DEGREE OF SCIENTIFIC INTEREST IN THE USE OF THE SUBSTANCE, AND • THE COHERENCE OF SCIENTIFIC ASSESSMENTS. CTD 3.2 (b) : BIBLIOGRAPHY “SHOULD COVER ALL ASPECTS OF THE SAFETY AND/OR EFFICACY ASSESSMENT AND MUST INCLUDE OR REFER TO A REVIEW OF THE RELEVANT LITERATURE, TAKING INTO ACCOUNT PRE-AND POST-MARKETING STUDIES AND PUBLISHED SCIENTIFIC LITERATURE CONCERNING EXPERIENCE IN THE FORM OF EPIDEMIOLOGICAL STUDIES AND IN PARTICULAR OF COMPARATIVE EPIDEMIOLOGICAL STUDIES”. “ WELL-ESTABLISHED USE” DEMONSTRATION (WEU) (2) ANNEX 1 TO CD 2001/83 EC AMENDED BY CD 2003/63 (25.06.2003) CTD 3.2 (c) : MISSING INFORMATION : JUSTIFICATION MUST BE GIVEN CTD 3.2 (d) : NON-CLINICAL AND /OR CLINICAL OVERVIEWS MUST EXPLAIN RELEVANCE OF ANY DATA SUBMITTED WHICH CONCERN A PRODUCT DIFFERENT FROM THE PRODUCT INTENDED FROM MARKETING. CTD 3.2 (e) : POST-MARKETING EXPERIENCE WITH OTHER PRODUCTS CONTAINING THE SAME CONSTITUENTS IS OF PARTICULAR IMPORTANCE AND APPLICANTS SHOULD PUT A SPECIAL EMPHASIS ON THIS ISSUE. DIRECTIVE 2004 / 24 / EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (2) * NEW LEGAL BASIS AND PROCEDURE • SIMPLIFIED REGISTRATION OF TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) UNDER ARTICLE 16A DOES NOT APPLY IN CASE THE “TRADITIONAL” HERBAL PRODUCT FULFILS THE CRITERIA FOR A FULL MARKETING AUTHORISATION • APPLICATION TO THE COMPETENT AUTHORITY OF THE MEMBER STATE (MS) • TIMEFRAME FOR IMPLEMENTATION - 210 DAYS REVIEW - MEMBER STATES : MAX. 18 MONTHS - EMEA : MAX. 18 MONTHS (STARTING ON 22.09.2004) - THMPs ALREADY ON THE MARKET IN THE MS : COMPLIANCE WITHIN 7 YEARS (04.2011). DIRECTIVE 2004 / 24 / EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (3) * SCOPE • INDICATION (S) EXCLUSIVELY APPROPRIATE TO THMPs AND DESIGNED FOR USE WITHOUT SUPERVISION OF A MEDICAL PRACTITIONER FOR DIAGNOSIS, PRESCRIPTION OR MONITORING OF TREATMENT • SPECIFIED STRENGTH AND POSOLOGY • ONLY ORAL OR EXTERNAL USE AND INHALATION • PERIOD OF TRADITIONAL USE : 30 YEARS (15 YEARS IN AND 15 YEARS OUTSIDE THE EU), UNLESS OTHERWISE DECIDED BY THE HMPC • VITAMINS AND MINERALS MAY BE ADDED IF THEIR ACTION IS ANCILLARY TO THE HERBAL CONSTITUENT(S) DIRECTIVE 2004 / 24 / EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (4) * DOSSIER REQUIREMENTS • ADMINISTRATIVE DOSSIER : APPLICATION FORM, EXPERT REPORTS, SPC • PHARMACEUTICAL DOSSIER : IDENTICAL TO A “ FULL” MARKETING AUTHORISATION • BIBLIOGRAPHIC OR EXPERT EVIDENCE THAT THE PRODUCT OR A CORRESPONDING MEDICINAL PRODUCT HAS BEEN IN MEDICINAL USE FOR AT LEAST 30 YEARS ( NOT NECESSARY IF LISTED OR MONOGRAPH) • BIBLIOGRAPHIC REVIEW OF SAFETY DATA TOGETHER WITH AN EXPERT REPORT (NOT NECESSARY IF LISTED OR MONOGRAPH) • MS MAY REQUEST FURTHER SAFETY DATA, IF NECESSARY DIRECTIVE 2004 / 24 / EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (6) * TASKS OF THE HERBAL COMMITTEE (HMPC) • ESTABLISH A LIST OF HERBAL SUBSTANCES, PREPARATIONS AND COMBINATIONS THEREOF FOR USE IN THMPs • ESTABLISH COMMUNITY HERBAL MONOGRAPHS FOR WELL-ESTABLISHED MARKETING AUTHORISATIONS OR TRADITIONAL REGISTRATIONS OF HMPs. MONOGRAPHS SHALL BE USED AS THE BASIS FOR ANY APPLICATION • AT THE REQUEST OF A MS DRAW UP AN OPINION ON THE ADEQUACY OF THE EVIDENCE OF THE LONG-STANDING USE • BE RESPONSIBLE FOR ARBITRATION / REFERRAL PROCEDURES ON THMPs • GIVE AN OPINION ON OTHER MEDICINAL PRODUCTS CONTAINING HERBAL SUBSTANCES REFERRED TO THE EMEA/CHMP DIRECTIVE 2004/24/EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (7) * POST- AUTHORISATION ACTIVITIES PHARMACOVIGILANCE REQUIREMENTS MANUFACTURING AND IMPORT PROVISIONS VARIATIONS - TAKING INTO TECHNICAL PROGRESS INSPECTION ACTIVITIES GMP COMPLIANCE WITH EU PHARMACOPOEIA MONOGRAPHS PHARMACOVIGILANCE DIRECTIVE 2004/24/EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (8) * LABELLING AND USER PACKAGE LEAFLET SHALL CONTAIN A STATEMENT “THE PRODUCT IS A TRADITIONAL HERBAL MEDICINAL PRODUCT FOR USE IN SPECIFIED INDICATION(S) EXCLUSIVELY BASED UPON LONG STANDING USE” “THE USER SHOULD CONSULT A DOCTOR OR QUALIFIED HEALTH CARE PRACTIONER IF THE SYMPTOMS PERSIST DURING THE USE OF THE PRODUCT OR IF ADVERSE EFFECTS NOT MENTIONED IN THE PACKAGE LEAFLET OCCUR - A MS MAY REQUIRE TO MENTION THE NATURE OF THE TRADITION IN QUESTION * ADVERTISEMENT SHALL CONTAIN THE STATEMENT “TRADITIONAL HERBAL MEDICINAL PRODUCT FOR USE IN SPECIFIED INDICATION(S) EXCLUSIVELY BASED UPON “LONG STANDING USE” OVERVIEW LEGAL FRAMEWORK FOR HERBAL MPS IN THE EU LEVEL OF EVIDENCE Legal basis Medicinal use Conditions New product Art. 8.3(i) 2001/83/EC “Complete application” Art. 10.1(a)(ii) 2001/83/EC ”Bibliographi cal application” < 10 years Major claim 10 years + demonstration of “well established use” Minor claim Minor Art. 16.a of the conditions proposed SelfDirective on 30 medication Traditional years Herbal + demonstration of Medicinal “traditional use” Products Fraudulent or misleading claims A B C T X Labelling Serious and minor diseases Herbal medicinal Possible intervention of product for... (treatment, medical prevention ...) practitioner No restrictions regarding strength, posology, route of administration Limited route of administration., strength & posology Traditionally used for… (no or limited scientific evidence) FRIAS, 2004 DIRECTIVE 2004/24/EC ON TRADITIONAL HERBAL MEDICINAL PRODUCTS (THMPs) (9) * PERSPECTIVES PURPOSE TO ESTABLISH A HARMONISED LEGISLATION FRAMEWORK FOR TRADITIONAL HERBAL MEDICINAL PRODUCTS IN EUROPE IMPLEMENTATION IN ORDER TO PROMOTE THIS HARMONISATION MS SHOULD RECOGNISE NATIONAL REGISTRATIONS OF THMPs GRANTED BY ANOTHER MS BASED ON COMMUNITY HERBAL MONOGRAPHS LIMITATIONS BORDERLINE MEDICINE-FOOD PRODUCTS - THE DIRECTIVE ALLOWS NON-MEDICINAL HERBAL PRODUCTS TO BE REGULATED UNDER FOOD LEGISLATION IN THE COMMUNITY - HERBAL PRODUCTS THAT ARE USED FOR HEALTH ENHANCEMENT/MAINTENANCE, REDUCTION OF RISK FACTOR FOR A DISEASE/ DISORDER/CONDITION OR GENERAL PROMOTION OF WELL-BEING/ FUNCTION OF THE HEALTHY BODY/ORGAN WILL PROBABLY BE REGULATED WITHIN THE AREA OF FOOD SUPPLEMENTS EUROPEAN DIRECTORATE FOR THE QUALITY OF MEDICINE (EDQM) www.edqm.org EUROPEAN PHARMACOPOEIA * GROUPS OF EXPERTS 13A AND 13B : PHYTOCHEMISTRY • SETTING STANDARDS FOR * * * * * PRODUCTION OF HERBAL DRUGS PHARMACEUTICAL INDUSTRY QUALITY CONTROL LABORATORIES REGULATORY AUTHORITIES COMMUNITY PHARMACISTS • MONOGRAPHS (TILL 2003) * HERBAL DRUGS : PUBLISHED : 106 UNDER STUDY : 40 * HERBAL DRUG PREPARATIONS : PUBLISHED : 58 UNDER STUDY : 48 * CERTIFICATION OF SUITABILITY TO THE MONOGRAPHS OF THE EP PUBLIC COMMITTEE RESOLUTION AP-CSP (99) 4 • EXTENSION OF THE EDQM CERTIFICATION PROCEDURE TO HERBAL DRUG PREPARATIONS (MARCH, 2003). OVERVIEW OF HERBAL SUBSTANCES/PREPARATIONS CPMP/QWP/2819/00, CORR. EMEA/CVMP/814/00, CORR. EUR. PH. SUPPL. 2002 HERBAL DRUGS HERBAL SUBSTANCES (PLANTAE MEDICINALES) HERBAL DRUG PREPARATIONS HERBAL PREPARATIONS (PLANTAE MEDICINALES PRAEPARATORE) COMMINUTED OR POWDERED DRUGS EXTRACTS (EXTRACTA) ESSENTIAL OILS (AETHEROLEA) HERBAL MEDICINAL PRODUCTS HERBAL TEAS (PLANTAE AD PTISANAM) EXPRESSED JUICES PROCESSED EXUDATES TRADITIONAL HERBAL MEDICINAL PRODUCTS DIFFERENT TYPES OF HERBAL EXTRACTS * EUR. PH. 2002, SUPPL. 4.3 HERBAL PREPARATIONS (HP) HERBAL EXTRACTS (HE) • LIQUID • SOFT: SEMI-SOLID NOT NATIVE HPs • NATIVE (GENUINE) EXTRACTS CONSIST SOLELY OF GENUINE HERBAL EXTRACTABLE MATTER • NOT NATIVE EXTRACTS CONTAIN ALSO TECHNICAL EXCIPIENTS, EXCIPIENTS NEEDED FOR STANDARDISATION AND/OR EXTRACTION SOLVENTS. DRY NATIVE HPs NOT NATIVE HPs TYPE A : STANDARDIZED (ADJUSTED) EXTRACTS TYPE B1 : QUANTIFIED EXTRACTS TYPE B2 : OTHER EXTRACTS REFINED “EXTRACTS (“PURIFIED”, “ENRICHED” EXTRACTS) • DER = THE RATIO OF THE STARTING MATERIAL TO THE GENUINE EXTRACT (20:1 = 5% OF EXTRACTABLE MATTER) CLASSIFICATION OF PLANT CONSTITUENTS 1. THERAPEUTICALLY ACTIVE CONSTITUENTS CHEMICALLY DEFINED SUBSTANCES OR GROUPS OF SUBSTANCES WHICH, IN AN ISOLATED STATE, EXERT THE SAME OR SIMILAR THERAPEUTIC EFFECT AS THE TOTAL EXTRACT. 2. ACTIVE CONSTITUENTS (PHARMACAEUTICALLY RELEVANT CONSTITUENTS) CHEMICALLY DEFINED SUBSTANCES OR GROUPS OF SUBSTANCES WHICH, IN AN ISOLATED STATE, DO NOT EXERT THE SAME THERAPEUTIC EFFECT AS THE TOTAL EXTRACT, BUT WHICH ARE ACCEPTED TO CONTRIBUTE TO THE THERAPEUTIC ACTIVITY OF THE HERBAL DRUG PREPARATION. 3. MARKERS CHEMICALLY DEFINED SUBSTANCES OR GROUPS OF SUBSTANCES WHICH ONLY SERVE ANALYTICAL PURPOSES. EXAMPLES: SILYMARIN, AESCIN, ANTRAQUINONES, ALKALOIDS, CARDIAC GLYCOSIDES, ... EXAMPLES: HYPERICINS, PROCYANIDINES, FLAVONOIDS. 3.1. CHARACTERISTIC MARKERS CHARACTERISTIC FOR THE RESPECTIVE GENUS OR FAMILY OF THE PLANT, SUITABLE FOR IDENTIFICATION, TESTS AND ASSAY (E.G. BATCH-TO-BATCH CONTROL). EXAMPLES: VALERENIC ACIDS, ECHINACOSIDE, ELEUTHEROSIDES B AND E. 3.2. UBIQUITOUS MARKERS OCCUR UBIQUITOUSLY IN PLANTS, SUITABLE FOR ASSAY (E.G. BATCH-TO-BATCH CONTROL). EXAMPLES: CHLOROGENIC ACID, RUTIN. NOTE FOR GUIDANCE ON SPECIFICATIONS : TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR HERBAL SUBSTANCES, HERBAL PREPARATIONS AND HERBAL MEDICINAL PRODUCTS (CPMP/QWP/2820/00/EMEA/CVMP/815/00) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 1. 2. 3. GENERAL CONCEPTS CHARACTERISATION DESIGN AND DEVELOPMENT CONSIDERATIONS PHARMACOPOEIAL TESTS AND ACCEPTANCE CRITERIA PERIODIC/SKIP TESTING RELEASE VERSUS SHELF-LIFE ACCEPTANCE CRITERIA IN-PROCESS TESTS ALTERNATIVE PROCEDURES EVOLVING TECHNOLOGIES REFERENCE STANDARD STATISTICAL CONCEPTS GUIDELINES SPECIFICATIONS : DEFINITION AND JUSTIFICATION UNIVERSAL TESTS/CRITERIA HERBAL SUBSTANCES HERBAL PREPARATIONS HERBAL MEDICINAL PRODUCTS SPECIFIC TESTS/CRITERIA HERBAL SUBSTANCES HERBAL PREPARATIONS HERBAL MEDICINAL PRODUCTS SPECIFICATIONS OF HERBAL SUBSTANCES NOTE FOR GUIDANCE ON SPECIFICATIONS: TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR HERBAL SUBSTANCES (CPMP/QWP/2820/00/EMEA/CVMP/815/00) BOTANICAL CHARACTERISTICS BIOLOGICAL/GEOGRAPHICAL VARIATION - DEFINITION - CHARACTERS - IDENTIFICATION MACROSCOPY MICROSCOPY HERBAL SUBSTANCES PHYTOCHEMICAL CHARACTERS STABILITY OF CONSTITUENTS - KNOWN THERAPEUTIC CONSTITUENTS - TOXIC CONSTITUENTS IDENTIFICATION - TLC - CHEMICAL REACTIONS TESTS ASSAY - TOTAL ASH - IN HCL INSOLUBLE ASH - FOREIGN MATTER - EXTRACTABLE MATTER - PARTICLE SIZE - WATER CONTENT CULTIVATION/HARVESTING DRYING CONDITIONS PRE/POST HARVEST TREATMENTS - PESTICIDES - FUMIGANTS - MICROBIAL LEVELS - AFLATOXINS - HEAVY METALS ETC.. SPECIFICATIONS OF HERBAL DRUG PREPARATIONS NOTE FOR GUIDANCE ON SPECIFICATIONS: TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR HERBAL PREPARATIONS (CPMP/QWP/2820/00/EMEA/CVMP/815/00) PHYTOCHEMICAL CHARACTERISTICS IDENTIFICATION QUALITY OF HERBALSUBTANCES STABILITY OF CONSTITUENTS ASSAY HERBAL PREPARATIONS CHROMATOGRAPHIC PROFILE . HPLC/UV-DIODE-ARRAY . HPLC/MS . GC/MS . TLC-DENSITOMETRY METHOD OF PREPARATION FROM HERBAL DRUG DEFINITION - STATE : SOLID, LIQUID ETC. - RATIO HERBAL DRUG TO HERBAL PREPARATION OR RATIO HERBAL SUBSTANCES TO EXTRACTION SOLVENT CHARACTERS SAFETY AND EFFICACY CONSIDERATIONS BATCHES USED IN PRECLINICAL/CLINICAL TESTING MICROBIAL STABILITY DRYING CONDITIONS - MICROBIAL LEVELS - RESIDUAL SOLVENTS IN EXTRACTS - WATER CONTENT - AFLATOXINS - HEAVY METALS - PESTICIDES - FUMIGANTS ETC. SPECIFICATIONS OF FINISHED HERBAL MEDICINAL PRODUCTS NOTE FOR GUIDANCE ON SPECIFICATIONS:TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR HERBAL MEDICINAL PRODUCTS (CPMP/QWP/2820/00/EMEA/CVMP/815/00) DESCRIPTION OF DOSAGE FORMS IDENTIFICATION . CHROMATOGRAPHIC PROFILE ASSAY SPECIFIC TESTS TABLETS AND HARD CAPSULES - DISSOLUTION/DESINTEGRATION - HARDNESS/FRIABILITY - UNIFORMITY OF DOSAGE UNITS - WATER CONTENT - MICROBIAL LIMITS QUALITY OF HERBAL PREPARATIONS HERBAL MEDICINAL PRODUCTS MANUFACTURING PROCESS . TEMPERATURE EFFECTS . RESIDUAL SOLVENTS . ORGANIC AND INORGANIC IMPURITIES . MICROBIAL LIMITS ORAL LIQUIDS - UNIFORMITY OF DOSAGE STABILITY OF THE ACTIVE CONSTITUENTS FORMULATION/PACKAGING SAFETY AND EFFICACY CONSIDERATIONS BACTHES USED IN PRECLINICAL/CLINICAL TESTING - pH.; WATER CONTENT; SPECIFIC GRAVITY - MICROBIAL LIMITS : ANTIMICROBIAL, AND ANTIOXIDATIVE PRESERVATIVE CONTENT - EXTRACTABLES : ALCOHOL CONTENT - DISSOLUTION : PARTICLE SIZE DISTRIBUTION - REDISPERSIBILITY : RECONSTITUTION TIME ANALYTICAL DEVELOPMENT OF HERBAL PREPARATIONS AND HERBAL MEDICINAL PRODUCTS IDENTITY PURITY DURING DEVELOPMENT ANALYSIS METHODS ASSAY ACTIVE INGREDIENT SPECIFICATION AT REGISTRATION TIME TEST PROCEDURE (detailled description) ANALYTICAL VALIDATION VALIDATION OF EACH TEST PROCEDURE PRECISION ACCURACY DETAILLED DESCRIPTION INCLUDING DOCUMENTS e.g. : . chromatograms . spectra . linearity VALIDATION MUST PROVE THE . recovery rates from spiked ACCURACY OF TEST RESULTS samples VALIDATION OF ANALYTICAL METHODS 1. WHICH VALIDATION CHARACTERISTICS HAVE TO BE VALIDATED? ACCORDING TO ICH (INTERNATIONAL CONFERENCE OF HARMONISATION) TYPES OF ANALYTICAL PROCEDURE IDENTIFICATION TESTING FOR IMPURITIES QUANTITATIVE ASSAY DISSOLUTION (MEASUREMENT ONLY) CONTENT/POTENCY LIMIT CHARACTERISTICS ACCURACY PRECISION : REPEATIBILITY INTERMEDIATE PRECISON SPECIFICITY (2) DETECTION LIMIT QUANTITATION LIMIT LINEARITY RANGE - + - + + - + + (1) + - (3) + + + - + + (1) + * * - + + - + + - : SIGNIFIES THAT THIS CHARACTERISTIC IS NORMALLY NOT EVALUATED + : SIGNIFIES THAT THIS CHARACTERISTIC IS NORMALLY EVALUATED (1) IN CASES WHERE REPRODUCIBILITY HAS BEEN PERFORMED, INTERMEDIATE PRECISION IS NOT NEED (2) LACK OF SPECIFICITY OF ONE ANALYTICAL PROCEDURE COULD BE COMPENSATED BY OTHER SUPPORTING ANALYTICAL PROCEDURE(S) (3) MAY BE NEEDED IN SOME CASES PRIMARY REFERENCE STANDARDS PLANT PREPARATION ISOLATION FULL DESCRIPTION INCLUDING PURIFICATION STEPS TO PROVE THE STRUCTURES CHARACTERIZATION IDENTITY TEST RESULTS OF ALL SPECIFIED QUALITY CHARACTERISTICS AND, IF RELEVANT, FOR PHYSICOCHEMICAL CHARACTERISTICS PURITY TEST RESULTS OF ALL POSSIBLE IMPURITIES ORGANIC IMPURITIES WATER CONTENT INORGANIC IMPURITIES ASSAY FULL DESCRIPTION INCLUDING TEST RESULTS E.G. 100% MINUS H2O AND INORGANIC IIMPURITIES SUM OF PEAKS =100% AREA MINUS AREA OF ALL IMPURITIES “AS IS”- ASSAY (VALUE) REFERENCE VALUE FOR WORKING STANDARD (SECONDARY) CHROMATOGRAPHIC PURITY - DIFFERENTIAL SCANNING CALORIMETRY - PHASE SOLUBILITY ANALYSIS MOLECULAR PURITY MARKETING AUTHORIZATION OR REGISTRATION OF HMPs MARKETING AUTHORIZATION REGISTRATION PHARMACOVIGILANCE APPLIES TO REGISTERED AND TO AUTHORIZED HMPs CONSUMER INFORMATION; LABELLING; ADVERTISING EFFICACY NEW TRIALS BIBLIOGRAHIC SAFETY NEW TESTS TRADITIONAL USE EXPERT REPORT BIBLIOGRAPHIC BIBLIOGRAPHIC NEW TESTS QUALITY CONTROL GOOD MANUFACTURING PRACTICES GOOD AGRICULTURE AND COLLECTION PRACTICES NEW N WEU TRADITIONAL MAY BE REPLACED BY A MONOGRAPH OR THE LIST FROM THE HMPC IN REGISTRATIONS IDENTICAL FOR MARKETING AUTHORIZATIONS AND REGISTRATIONS MARKETING AUTHORIZATION OR REGISTRATION OF ANTIMALARIALS FROM ARTEMISIA ANNUA L. NEW WEU TRADITIONAL ARTEMISININ • DIHYDROARTEMISININ • ARTHEMETHER • ARTHEETHER • ARTELINATE • ARTESUNATE ... ARTHEMETHER ... AND LUMIFANTRINE OR MEFLOQUINE ... ARTESUNATE ... AND SULFAMETHOXYPYRAZINE/ PYRIMETHAMINE ... ARTEMISIA ANNUA EXTRACT AND OTHER ANTIMALARIALS ARTEMISIA ANNUA EXTRACT SUMMARY • THE HMPC IS FULLY OPERATIONAL SINCE NOVEMBER 2004. • FIRST DECISIONS ON PROCEDURAL ASPECTS ALREADY PUBLISHED IN JANUARY 2005. • POSITIVE ATTITUDE AND COMMITMENT OF ALL EXPERTS. • THE HMPC IS COMMITTED TO DELIVER SCIENTIFICALLY VALID RESULTS, AS FAST AS POSSIBLE AND IN FULLY TRANSPARENT WAY. • BOTH, TRADITIONAL AND WELL-ESTABLISHED HERBAL MEDICINAL PRODUCTS WILL BENEFIT FROM THESE ACTIVITIES. • NEW LEGAL AND SCIENTIFIC AREAS ARE TO BE EXPLORED • SIGNIFICANT WORKLOAD IN PREPARING THE GROUNDS FOR ROBUST RESULTS. • RESOURCES FROM EMEA AND FROM MEMBER STATES NEED TO BE CLEARLY ALLOCATED. K. KELLER, 2006