Chapter I
advertisement
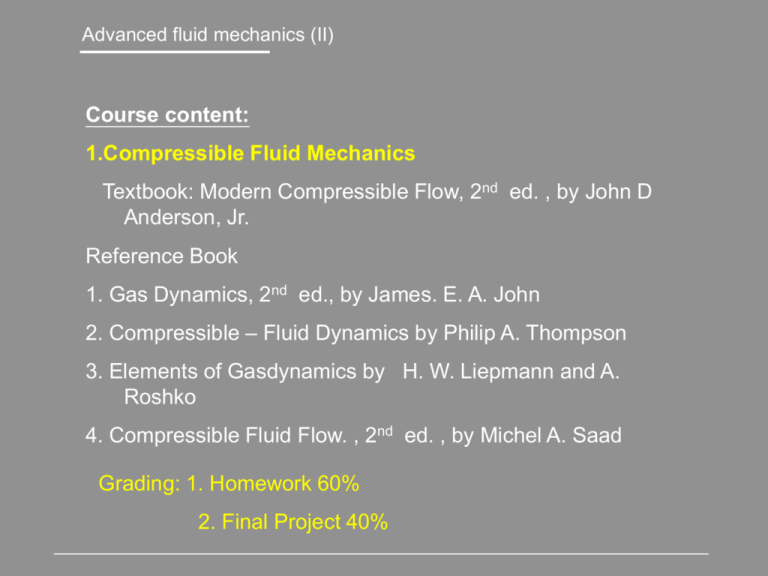
Advanced fluid mechanics (II) Course content: 1.Compressible Fluid Mechanics Textbook: Modern Compressible Flow, 2nd ed. , by John D Anderson, Jr. Reference Book 1. Gas Dynamics, 2nd ed., by James. E. A. John 2. Compressible – Fluid Dynamics by Philip A. Thompson 3. Elements of Gasdynamics by H. W. Liepmann and A. Roshko 4. Compressible Fluid Flow. , 2nd ed. , by Michel A. Saad Grading: 1. Homework 60% 2. Final Project 40% Chapter I 1. Introduction and Review of Thermodynamics What is Compressible Flow? 1. Const 2. Energy transformation and temperature change are important considerations → Importance of Thermodynamics e.q Flow of standard sea level conditions, M 2 Specific internal energy 8314 .300 RT 28 i CvT 2.2 105 J / kg 1 0.4 Specific kinetic energy 2 U k 2 22 U 2a RT 2 i 0(k ) 2 2 1.4 8314 300 2.5 105 J / kg 28 Chapter I 1.1 Definition of Compressible Flow Incompressible flow → compressibility effect can be ignored. ν is the specific volume & 1/ v Compressibility of the fluid 1 dv v dp Note: dp(+) → dv(-) Physical meaning: the fractional change in volume of the fluid element per unit change in pressure Chapter I 1 v )T ...... v p …. Isothermal compressibility 1 v ) S ...... v p …..isentropic compressibility (speed of sound) T ( S ( Compressibility is a property of the fluid Liquids have very low values of e.g T for water = 5 1010 m2 / N at 1atm Gases have high e.g T for air =10-5 m2/N at 1 atm, Alternate form of v 1 1 d dp d dp Chapter I For most practical problem d 5% compressible General speaking Ma >0.3 → Compressible effect can not be ignored Ma < 0.3 → Incompressible flow Chapter I 1.2. Regimes of compressible flow Subsonic flow Flow is forewarned of the presence of the body Streamline deflected far upstream of the body Chapter I Transonic flow Mis less than 1 , but high enough to produce a pocket of locally supersonic slow If M is increased to slightly above 1 , the λ shock will move to the trailing edge of the airfoil , and bow shock appears upstream of the leading edge. Loosely Defined as the “ Transonic regime” (Highly unstable) Chapter I Supersonic Flow M 1 Everywhere (We will mostly focus on this regimes) T , p, Behind the shock + Parallel the free stream flow is not forewarned of presence of the body until the shock is encountered + Both flow of upstream of the shock and downstream of the shock are supersonic + Dramatic physical and mathematical difference between subsonic and supersonic flows. Chapter I Hypersonic Flow T , p, High enough to excite the internal modes of energy dissociate or even ionize the gas. Real gas effect !!! Chemistry comes in Chapter I Incompressible flow is a special case of subsonic flow limiting case M 0 V 0 V M a Trivial , no flow a a , 0 For incompressibility Viscous Flows Flow can be also be classified as inviscid Viscous flow: + Dissipative effects : Viscosity, thermal conduction, mass diffusion…. + Important in regions of large gradients of V, T and Ci e.g. Boundary layer Chapter I Inviscid flows: - ignore dissipative effects outside of B.L (We will treat this kind of flow ) Also consider the gas to be “ Continuum ” Mean free path Kn L 1 1.3 A Review of Thermodynamics 1.3.1 Ideal gas – intermolecular force are negligible p RT pv RT p nkT R* R - specific gas constant M 8314 (J/kg.mole.k) Molecular weights Boltzmann constant = 1.38 1023 J / K For air at standard conditions R 8314 R 287 J /(kg.K ) 1716( ft.lb) /( slug. 0 R) M 28.9 L d L > 10d , for most compressible flows Isothermal compressibility 1 v ( )T v p RT v RT v 1 v 1 v ;( ) 2 T ( )T p p p p v p p T Chapter I 1.3.2. Internal Energy and Enthalpy -Translational -Rotational No of collisions > 5 → equilibrium -Vibration : No of collisions > 0 (100 ) → equilibrium Add one more time scale or length scale -Electronic excitation + nuclear Statistical Thermodynamics + Quantum mechanics If the particles of the gas (called the system) are rattling about their state of “maximum disorder”, the system of particle will be in equilibrium. Chapter I Return to macroscopic view continuum Let e be specific internal energy Let h be specific enthalpy h e pv For both a real gas and a chemically reacting mixture of perfect gases. e e(T , v) h h(T , p) Thermally perfect gas e eT h hT de Cv dt dh C p dt Cv (T ), C p (T ) Chapter I Calorically perfect gas e CvT h C pT Will be assumed in the discussion of this class Ratio of specific heat , Cp C p , Cv are const → cons tan t γ =1.4 for a diatomic gas Cv γ =5/3 for a monatoinic gas Air, T<1000 K – Calorically perfect gas O2, N2, 1000<T<2500 – Thermally perfect gas Vibrational excited O2 dissociate 2500<T<4000 K N2 dissociate T>4000K Consider caloriacally perfect gas + thermally perfect gas e cvT p RT h c pT Cp Cv Note: he pv R T T h Cp T p e Cv T v specific heat at constant pressure specific heat at constant volume Chapter I Perfect gas Cv , C p , cons tan t Ideal gas e e(T ) h h(T ) p RT Cp R R Cp Cv R; Cp , Cv Cv 1 1 1.3.3. First law of the thermodynamics -Conservation of Energy Consider a system, which is a fixed mass of gas separated from the surroundings by a flexible boundary. For the time being, assume the system is stationary, i.e., it has no directed kinetic energy q w de An incremental amount of heat added to the system across the boundary e is state variable, de is an exact differential depends only on the initial and final states of the system The work done on the system by the surrondings Chapter I For a given de , there are in general an infinite different ways (processes) of q w We will be primarily concerned with 3 types of processes: 1. Adiabatic process q 0 2. Reversible process – no dissipative phenomena occur, i.e,. Where the effects of viscosity, thermal conductivity, and mass diffusion are absent w pdv (see any text on thermodynamic) 3. Isentropic process - both adiabatic & reversible 2nd law of thermodynamic Chapter I 1.3.4 Entropy and the Second Law of Thermodynamic Define a new state variable, the entropy, ds qrev or T ds q T siirev The actual heat added/T, siirev 0 A contribution from the irreversible dissipative phenomena of viscosity thermal conductivity, and mass diffusion occurring within the system These dissipative phenomena “ always” increase the entropy For a reversible process ds q T If the process is adiabatic, q 0 2nd law ds sirrev 0 In summary, the concept of entropy in combination with the 2nd law allow us to predict the direction that nature takes. Chapter I Assume the heat is reversible, q qrev qrev Tds q pdv de Tds de pdv 1st law becomes h e pv dh de pdv vdp Tds dh vdp For a thermally perfect gas, ds C p dh C p dT dT vdp T T If the gas also obey the ideal gas equation of state p RT ds C p dT dp R T p T2 dT P2 R ln Note Integrate s2 s1 Cp T P 1 T1 C p C p (T ) Chapter I If we further assume a calorically perfect gas, T2 P2 s2 s1 Cp ln R ln T1 P1 T2 v2 Cv ln R ln T1 v1 C p Cv R Tds de pdv 1.3.5. Isentropic realtions For an adiabatic process q 0 and for a reversible process dsirrev 0 Hence, from eq ds q dsirrev 0 ,i.e., T the entropy is constant. s2 s1 Chapter I T P P T C /R T 0 C p ln 2 R ln 2 2 ( 2 ) p ( 2 ) 1 T1 P1 P1 T1 T1 1 T2 v2 v2 T2 Cv / R T2 1 0 Cv ln R ln ( ) ( ) T1 v1 v1 T1 T1 2 T2 1 ( ) 1 T1 1 P2 T ( 2 ) ( 2 ) 1 P1 1 T1 Outside B.L-Isentropic relations prevail e.g. T=1350K P=? T=2500 K P=15atm M=12, Cp=4157 J/kg.K P2 T ( 2 ) 1 0.0248 P1 T1 Cp Cv 1.2 ; Cv C p R 4157 8314 3464 J / kg.k 12 Chapter I 1.3.6. Aerodynamic forces on a Body Main concerns : Lift & drag Forces on a body of airfoil -Surface forces: pressure shear stress -Body forces : gravity ; electric-magnetic Sources of aerodynamic force, resultant force and its resolution into lift and drag Chapter I Let n & m be unit vectors perpendicular and parallel, respectively to the element ds, dF pnds mds F dF pds mds Lift L is the component of F perpendicular to the relative wind V Drag D is the component of F parallel In our plot. L// y, D// L pds y x mds pds (resonable ) y inviscid y Chapter I D pds x Pressure drag -> wave drag, e.g slender supersonic shapes with shock waves mds x Skin friction drag -We consider only inviscid flows and both pressure and skinfriction drags are important -In the most cases, we can not predict the drag accurately For blunt bodies, Dp dominates For streamlined bodies, Dskin dominates with shock wave, Dwave drag dominate and Dskin can be neglected D can be predicted reasonably