Photoelectric Effect – Virtual Lab
advertisement

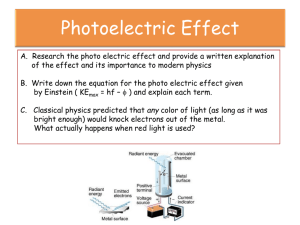
Name: _________________________________________ Date: ______________ Section: _____________________ 11.1 – PHOTOELECTRIC EFFECT – VIRTUAL MINI-LAB Objectives: WWBAT… Explain how the photoelectric effect experiment works and why a photon model of light is necessary to explain the results. Study the effect of intensity of light on photoelectric experiment. Calculate the wavelength of light, the work function of the metal, or the stopping potential, if given the other two. Background Information The photoelectric effect is one of the key experiments that supported early quantum theory. Light, prior to the early 20th century, was considered to be a wave phenomenon. In most ways, this idea reflects reality well—for instance, light is bendable when passed through a lens. The energy of a wave is given by amplitude of the wave squared, so a light wave of a certain frequency should be able to have any value for energy as long as there is a bright enough light source. However, when red light was shone on a metal surface, no electrons were ejected even when the brightest red light sources were used. On the other hand, when blue light was shone on the same metal surface electrons were ejected even when the source of light was weak (and brighter blue lights ejected more electrons) How could this be? The energy didn’t seem to depend on the amount of light hitting the metal but instead the frequency of light that hit the metal. Planck put us on the path leading out of this thicket of confusion when he theorized that light and other forms of energy comes in “packets” or discreet “bundles”. Light, in this theory, is considered to be a particle, which we now call a photon. The photoelectric effect was explained by Einstein when he conjectured that Planck’s bundles of energy (i.e. photons) were “knocking loose” the electrons—but only if the photons had enough energy to do the job (a two year old isn’t able to knock a football player off his feet, but a bull undoubtedly could). Einstein’s ideas gave further support to the theory that light energy really is not continuous with infinitely small increments of change (a wave), but is in fact made up of quanta called photons. Today’s lab involves a simulation of the photoelectric effect. You will be checking various metals for the point at which they begin to shed electrons, based on a specific threshold frequency—the exact point when the photons have enough energy to knock the electrons loose. This energy is called the work function (ɸ) for the metal. Different metals hold on to their electrons more strongly or weakly due to atomic structure, so the work function for various metals varies. The formula for calculating ɸ is as follows: 𝐾𝐸 = ℎ𝑓 − ɸ Where h is Planck’s constant f is the frequency of the light KE is the kinetic energy of the ejected electron ɸ is the work function The kinetic energy of the electron refers to its actual movement once ejected. KE can effectively be ignored if we just reach the amount of energy to loosen the electron but not get it moving (KE in these circumstances will essentially have a value of zero). You will be trying to achieve the lowest possible speed for the electrons you eject from the virtual metal surface. W can be obtained by calculating frequency and using Planck’s constant. The work function will be in joules, so in order to compare to published lists of work function values—which are in electron volts—your final value will require conversion into this unit. Procedure The “equipment” you will be working with looks like this: 1. Bring up the internet and go to the following site: http://phet.colorado.edu/en/simulation/photoelectric 2. Click “Run now” (your JavaScript will have to be enabled). 3. Ensure that the Target in the upper right hand corner is sodium. In the panel in the right hand side, also check the boxes that state, “Only show highest energy electrons,” and “Electron energy vs. Light Frequency.” 4. Keep battery voltage at 0, and turn the light source on to a very low intensity (10%). 5. Increase the wavelength and observe what happens to the speed of the electrons and number of electrons ejected from the metal. Note this observation in Data Table A. 6. Now increase the intensity of the light and observe what happens to the speed of the electrons and number of electrons ejected from the metal. Note this observation in Data Table A. 7. Now set the intensity to 50%. Adjust wavelength to a value which just allows electrons to leave the surface at a kinetic energy of approximately 2 eV (you can determine this from the graph on the right side of the screen). Record this wavelength and the kinetic energy of the electron in Data Table B. 8. Change the metal in the upper right hand corner. Repeat Step #7 for all of the metals, including the mystery metal. 9. For each metal, calculate the work function in electron volts. Complete these calculations in the box labeled “Work Function Calculations.” Keep in mind the following constants and conversions: c = 3.0 x 108 m/s h = 6.63 x 10-34 Js mass of electron = 9.1 x 10-31 kg 1 electron volt = 1.6 × 10-19 J Charge of an electron = 1.6 x 10-19 C 10. Predict the kinetic energy and stopping potential of an electron emitted from sodium by 400 nm light. Complete these calculations in the box labeled “KE and Stopping Potential Calculations”. 11. Now set the target to Sodium, the wavelength to 400 nm, and the intensity to 100%. Set the voltage of the battery to the negative of your predicted stopping potential (i.e. if Vs = 7 V, set the voltage to -7). Observe how far the electrons travel (halfway, ¾ way, etc.) before stopping, if they stop. Record your observation in Data Table A. 12. Complete the concluding questions for this experiment. Data and Calculations Data Table A Action Increasing frequency of light Observation Increasing intensity of light Setting battery equal to stopping potential Data Table B Metal Sodium KE (eV) 2 2 2 2 Calculations for Work Function: Calculations for Stopping Potential: Wavelength (nm) Work Function Concluding Questions: 1) 200 nm light is shone on sodium metal and the sodium becomes positively charged. When 700 nm light is shone on sodium metal, however, the sodium metal remains neutral. Explain why this is the case. 2) During a photoelectric effect experiment, Johnny uses the electrons emitted from Zinc metal to create a current in a circuit. Johnny initially tests his circuit using blue light, and gets a current of 1.0 mA. a. If Johnny uses red light instead, what will happen to the current? Explain your answer. b. If Johnny increases the intensity of the blue light he’s using, what will happen to the current? Explain your answer. 3) Marjorie is trying to determine the work function of a particular metal. She shines red, green, and yellow light on the metal, but Marjorie does not observe any electrons being emitted. Marjorie concludes that the metal is not photoactive, and therefore has no work function. Explain the error Marjorie made in designing her experiment, and why she may not have observed electrons being emitted. 4) Sam discovered that the threshold frequency for a particular metal is 500 THz. Determine the stopping potential for an electron emitted from this metal when 200 nm light is shone on it.