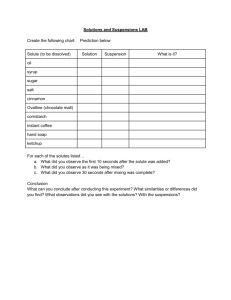
PPT - CABM Structural Bioinformatics Laboratory
advertisement
Biophysical Studies of Protein-DNA Interactions Solute and Salt Effects In Vitro and In Vivo Record Laboratory UW-Madison Departments of Chemistry and Biochemistry Probing large-scale conformational changes and other coupled processes in RNA polymerase, lac repressor, and IHF - DNA interactions (DNA wrapping and/or opening, protein folding) Ruth Saecker Carrie Davis Wayne Kontur Laurel Pegram Kirk vanderMeulen Melissa Anderson Mike Capp Junseock Koh Oleg Tsodikov (Harvard) Jill Holbrook (U. Heidelburg) Escherichia coli as an osmotic system; solute-biopolymer interactions in vivo and in vitro Scott Cayley Charles Anderson Mike Capp Jonathan Cannon Jiang Hong Irina Shkel Jeff Ballin Elizabeth Courtenay (MIT) Dan Felitsky (Scripps) Supported by the NIH ASA-Based Prediction or Interpretation of Solute Effects On Biopolymer Processes Solutes: Denaturants (e.g. urea, GuHCl) Osmolytes, Stabilizers (e.g.glycine betaine (GB)) Hofmeister Salts (e.g. KF, KGlu vs. KSCN, KI) Crystallization Agents (e.g. PEG, MPD, (NH4)2SO4) Processes: (∆ASA< 0) Folding, Helix Formation Dimerization, Assembly Crystallization, Precipitation Solute Series (Hofmeister ions, uncharged solutes): Anions: Sulfate, Phosphate, F, Glu, Ac, Cl, Br, I, SCN Cations: NR4, K, Na, GuH Uncharged: MPD, TMAO, GB, Pro, Glycerol, Formamide, Urea Solute effects arise from PREFERENTIAL INTERACTIONS (Timasheff): Solute and water compete for the biopolymer surface Preferential Accumulation of Solute: Solute-Biopolymer interactions more favorable than interactions of both species with water Local concentration of solute higher than bulk Preferential Exclusion of Solute (Preferential Hydration) Local concentration of solute lower than bulk To describe solute distribution: Schellman 1:1 solute: water competitive binding model Our solute partitioning model; partition coefficient Kp Kp = m3loc/m3bulk If Kp > 1, solute is accumulated; if Kp < 1, solute is excluded Preferential Accumulation and Exclusion Preferential interactions in principle are measurable by equilibrium dialysis. Preferential interaction coefficient is same as dialysis or Donnan coefficient. Although the dialysis analogy is useful conceptually, we find that vapor pressure osmometry (VPO) is more efficient and as accurate as dialysis as a method of characterizing preferential interactions Preferential Interaction Coefficient: H 2O H 2O (1) 3 1 3 T, , 1 Biopolymer (2) Solute Solute (3) m3 m2 m 3 m2 3 at dialy sis equilibrium Local/Bulk Model Bulk Local local bulk n3 n3 local n1 bulk n1 bulk = m3 m1 Local local n3 local local KP = (n3 / n1) bulk (n3 / n1) local = m3 n1 bulk m3 bulk / m = (ASA)(K – 1)b m P 1 3 1 3 where b1(ASA) = B1 = n1local/n2 > 0 accumulation < 0 exclusion Systems investigated to date: Solutes: E. coli osmolytes (GB, Pro, trehalose, KGlu) Denaturants (urea, GuHCl, GuHSCN) Hofmeister salts (KF, KCl, KBr, KI) Biopolymer Surfaces (ranging from nonpolar and uncharged to highly charged): Surface exposed on unfolding: Globular proteins (lac I HTH; 73% nonpolar, Alpha-helix essentially uncharged) DNA double helix Native protein surface (20-30% charged) Native DNA surface (44% charged surface) Quantifying Preferential Interactions of Solutes With Native Biopolymer Surface (Enriched in Charged and Polar Groups): Measure excess or deficit osmolality ∆Osm(m2,m3): From ∆Osm(m2,m3) determine effect of solute on biopolymer chemical potential (activity coefficient) From µ23, determine preferential interaction coefficient µ 3 which is approximately equal to equilibrium dialysis coefficient where At low solute concentration, intensive quantity (per unit of biopolymer surface) where Kp is solute partition coefficient and b1o is hydration (H20/A2) ∆Osm is proportional to m3 at constant m2 and increases with increasing m2 at constant m3 J. Cannon & M. Capp, submitted ‘04 is proportional to m3, not a function of m2, and much larger for BSA than for HEWL at a given m3 J. Cannon & M. Capp Urea is Weakly Accumulated Near Native BSA Surface; Betaine is Strongly Excluded (from anionic carboxylate oxygens) (J. Cannon & M. Capp) Preferential Interactions with B-DNA Urea is Neither Strongly Accumulated Nor Excluded from ds DNA; Betaine is Strongly Excluded (largely from anionic phosphate oxygens) J. Hong Quantifying Preferential Interactions of Solutes With Biopolymer Surface Exposed in Unfolding/Melting (Enriched in Uncharged and Nonpolar Groups): Measure or Tm as a function of solute concentration m3 For uncharged solutes (Wyman) (For Electrolyte solutes ) For uncharged solutes Interpret as for interaction of solute with biopolymer surface exposed in unfolding (u) At low solute concentration and dlnKobs/dm3 = “m-value”/RT = (Kp - 1)b1o(ASA)/ 55.5 “m-value” is the slope of a plot of -∆Gobso =RTlnKobs for unfolding or other biopolymer process vs. solute concentration lacI HTH as a Model System for Folding Studies • Small helix-turn-helix protein • Two state reversible equilibrium unfolding • Marginal stability; population not 100% in folded state even at temperature of maximum stability • Broad thermal and solute-induced transitions permit experimental study over wide ranges of temperatures and solute concentrations. Urea Induced Unfolding of lacI HTH Urea (M) Fraction Unfolded Fraction Unfolded 6 5 4 3 2 0 Temperature (C) Urea Molarity Felitsky et al., Biochemistry, ‘03 Betaine 0 4M Temperature (C) Fraction Unfolded Fraction Unfolded Betaine Effects on lacI HTH Stability Betaine Molarity ( Felitsky et al., Biochemistry,submitted) (Felitsky et al. 2003) Betaine has Qualitatively Different Interactions with Different Surfaces ASA or ASA 3/(m3ASA) x 103 -0.38 0.05 lacI HTH Unfolding (Felitsky, Cannon et al., 04) native lysozyme native bovine serum albumin Nonpolar 39% Charged 39% Other Polar 22% native DNA -0.47 0.17 -0.83 0.05 -1.82 0.12 (Hong, Cannon et al, 04) Glycine Betaine: Correlation of Exclusion with Anionic Biopolymer Surface (carboxylate, phosphate oxygens) Felitsky, ‘04 Urea: Correlation of Accumulation with Polar Amide Surface Deviations for highly anionic surfaces suggest modest exclusion of urea from vicinity of carboxylate and phosphate oxygens. Hong et al. ‘04 Applications Effect of Uptake of GB on Amount of Cytoplasmic Water and Growth Rate of Osmotically-Stressed E. coli Urea and GB as Probes of Coupled Folding or Unfolding and of Other Coupled Processes in the Steps of RNA Polymerase-Promoter Binding Osmotic Stress Reduces Growth Rate of E. coli 1.2 MBM+GB 1 1 Growth rate (hr -1 ) 1.2 0.8 0.8 LB 0.6 0.6 0.4 0.4 MBM 0.2 0.2 0 0 0 1 2 3 External Osmolality (Osm) (S. Cayley et al, ‘03) Glycine Betaine (GB) increases growth rate at high osmolality and therefore is a very effective osmoprotectant in E. coli Passive and Active Responses to Osmotic Stress •Initial (passive) response to osmotic stress: loss of water and turgor pressure Subsequent (active) response: accumulation of osmolytes, resulting in uptake of water Cayley et al, ‘03 Accumulation of GB Increases the Amount of Cytoplasmic Water Without Increasing the Total Amount of Osmolytes Propose that GB is a more efficient osmolyte than Kglu or trehalose because it is so highly excluded from anionic surface of DNA, RNA, and proteins. Steady State Amount of Cytoplasmic Water Decreases with Increasing Osmolality of Growth Cytoplasmic water ( l/mg DW) 2.4 1.9 +GB 1.4 -GB 0.9 0.4 0 1 2 Growth osmolality (Cayley et al., ‘03) •Accumulation of solutes does not prevent reduction in steady state amount of cytoplasmic water with increasing growth osmolality •Accumulation of betaine increases amount of cytoplasmic water at a given Osm Linkage of Growth Rate and Cytoplasmic Water 1.2 1 1 -1 Growth rate (h ) 1.2 0.8 0.8 0.6 0.6 0.4 0.4 0.2 0.2 0 0 0 50 100 150 Amount of cytoplasmic water (mol/mg dry weight) Cayley et al., ‘03 Summary of Results For the homologous series of surfaces exposed in unfolding globular proteins (with similar surface compositions and a wide range of ASA, values of for preferential interactions of urea and GuHCl are proportional to m3 and to ASA, and Kp is the same for all proteins in the series. Analysis of the exclusion of GB from different biopolymer surfaces indicates that GB is completely excluded (Kp = 0) from anionic (carboxylate, phosphate) oxygen surface and that hydration of this anionic surface is 2 layers of water (0.23 H20/A2). GB therefore drives biopolymer processes in which anionic surface is dehydrated. Urea accumulates at polar amide surface of proteins and nucleic acid bases (Kp = 1.8 if hydration is a monolayer); urea appears to be weakly excluded from anionic oxygen surface. Conclusion: Can quantitatively predict effects of urea, GB on biopolymer processes from structure (∆ASA; composition). In absence of structure, can interpret effects of urea, GB in terms of ∆ASA if assume a particular surface composition. CAN THIS BE EXTENDED TO OTHER SOLUTES AND PROCESSES?