Chapter 14 atmosphere - San Diego Miramar College
advertisement

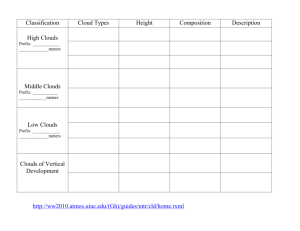
Chapter 14 – The Atmosphere The modern atmosphere • Two most abundant gases: – 78% N2 – 21% O2 • Less abundant gases (< 1%) – Argon – Water vapor – CO2 (only about .035%) • Non-gaseous components – water droplets – dust, pollen, soot and other particulates Fig. 17.6, p.437 Thermal Structure of Atmosphere: Troposphere • Extends to about 12 km (40,000 ft) elevation • All clouds & water vapor and most weather • Temperature decreases as elevation increases, because chief source of heat is radiated heat from the earth’s surface • Environmental Lapse rate: 6.4°C/1000m (3.5°F/1000ft) Lapse rate: the rate at which air temperature decreases with altitude. Tropopause: boundary between troposphere and stratosphere Thermal Structure of Atmosphere: Upper Layers • Stratosphere – heated primarily by solar radiation – Ozone (O3) layer absorbs UV energy, causing temperatures to rise – Above 55km (stratopause) temps fall again • Mesosphere – thin air (can’t absorb energy), very cold up to 80km • Thermosphere – above 80km, temps rise rapidly (to just below freezing!) Latent Heat • Phase change: change from a more ordered state to a less ordered one, or from a less ordered state to a more ordered one • Latent heat – energy stored or released during a phase change without temperature change. Latent Heat • Sometimes heat transfer causes a change of state – change from solid to liquid, or liquid to gas. This occurs without a change in temperature. Examples: – Water stores energy when it changes from a liquid to a gas, since it absorbs the energy, but does not change temperature. Evaporated of sweat stores heat energy from your body and carries it away. – Water releases energy when it changes from a gas to a liquid. This is the source of wind energy found in hurricanes. Humidity • H2O in air exists in 2 forms: vapor (invisible) and liquid e.g. rain, fog, clouds. • Saturation: Maximum quantity H2O vapor that air can hold. Additional H2O condenses into liquid water. • Saturation increases with temperature (hot air can hold more water vapor than cold air. • Conditions found on left side of curve cause water vapor to condense. Relative Humidity • Relative humidity (RH)- ratio of actual amount H2O vapor in air to the amount at saturation. • Often, as the days warms up, the air, more water will evaporate into the air (actual amount), but since the temperature has increased, so has the amount of water needed for saturation • Therefore, the RH stays about the same. RH = Grams water vapor in air x 100% Grams vapor at saturation Relative Humidity • What happens if the air now cools down? • Actual amount of water vapor in the air stays the same but amount needed for saturation decreases. • Therefore the RH increases. Relative Humidity • When the relative humidity reaches 100%, the air is saturated, regardless of actual amount of water vapor present. • At low temperatures, 100% RH means less water vapor than at higher temperatures. Dew Point Temperature RH = Grams water vapor in air x 100% Grams vapor at saturation • Dew point temperature – temperature at which RH = 100 %. • If saturated air cools below the dew point temperature, water vapor will condense into liquid. • At the dew point temperature, clouds, fog rain or dew appear. Solar Energy (Insolation) – Also called solar radiation, although NOT radioactive! – Composed of electromagnetic waves with different properties depending on wavelength, frequency • Longwave (low frequency): includes heat (infrared), radio waves • Shortwave (high frequency): includes visible light as well as ultraviolet, x rays, gamma rays • Electromagnetic spectrum – shows EM wavelengths by frequency and wavelength. Fig. 18-2, p.430 Shortwave solar energy • Much of the highest frequencies (x-rays, gamma rays) are absorbed by oxygen atoms in the thermosphere and O2 gas in the mesosphere. • Much ultraviolet (uv) absorbed by the ozone in the stratosphere. • Visible light waves (still considered shortwave) pass through atmosphere and are absorbed by earth. Solar Radiation in the atmosphere • Scattering and Reflection • Absorption, which is often accompanied by re-emission Reflection and Albedo • Reflection–electromagnetic radiation bouncing of from a surface without absorption or emission, no change in material or energy wavelength • Albedo – proportional reflectance of a surface – – – – – a perfect mirror has an albedo of 100% Glaciers & snowfields approach 80-90% Clouds – 50-55% Pavement and some buildings – only 10-15% Ocean only 5%! Water absorbs energy. Typical Albedos of Materials on the Earth Absorption and Emission • Absorption of radiation – electrons of absorbing material are “excited” by increase in energy – Increase in temperature; physical/chemical change – Examples: sunburn, cancer • Emission of radiation – excited electrons return to original state; radiation emitted as light or heat – Example: earth absorbs short wave radiation from sun (i.e. visible light) and emits longwave (infrared or heat) into the atmosphere Fig. 18-6, p.432 the Radiation Balance • Sun emits EM radiation of all wavelengths, but primarily shortwave (i.e. light). – Earth’s surface absorbs this energy – Most is re-emitted upward, as heat (longwave) • Greenhouse Effect – “greenhouse gases” (water vapor, carbon dioxide, methane, etc.) let shortwave energy pass, but absorb and longwave energy radiated upward by the Earth. – this longwave energy is re-radiated in all directions, some of it returning to the Earth’s surface. This is what keeps our atmosphere at a livable temperature of about 15 degrees C (59 degrees F). Cloud Formation • Clouds – made of water droplets and/or ice particles (not water vapor, which is invisible) • Cloud particles grow around a tiny solid surface; these are called condensation nuclei (singular nucleus). • Examples include dust, smoke, pollen, sea salt pollutant particulates. Cloud Formation • clouds form when the air is saturated (100% relative humidity) and H20 vapor condenses. • air must cooled to dew point for saturation to occur. • Large scale cooling occurs when an air mass is lifted to a higher level in the atmosphere, due to the Adiabatic Principle. The Adiabatic Principle • When a gas expands, it’s temperature decreases. • When a gas is compressed, it’s temperature increases. • Air expands when it goes up in altitude, where the air pressure is less. • Air compresses when it goes down, as air pressure increases. The Adiabatic Process The Adiabatic Process refers to a temperature change caused by a change in pressure. • Adiabatic (expansional) cooling – occurs when rising air expands due to decrease in air pressure. • Adiabatic (compressional) warming - sinking air compresses, due to increase in air pressure. another example of adiabatic warming – Santa Ana winds Adiabatic Lapse Rates Dry: 10°C/1000m Wet: approx 5°C/ 1000m <--- lifting condensation level Cloud Formation • Warm,moist air parcel gets “pushed up” and cools adiabatically. • If not saturated, cools at the dry lapse rate. • Starts rising because it is warmer and therefore less dense than it’s surroundings • gets “pushed” by some mechanism (e.g.cold front, mountains) to keep rising, since it cools faster than its environment. • As it cools down, its RH increases towards 100%. Lapse Rates Dry Adiabatic: 10°C/1000m Wet Adiabatic: approx 5°C/ 1000m Environmental: approx. 6.4°C/1000m Cloud Formation • Lifting Condensation Level: Altitude where the temperature of the parcel drops to the dew point temperature. At this temperature, RH = 100% and the parcel has become saturated. • Condensation begins around any condensation nuclei that are present, and tiny water droplets form. • “A Cloud is Born!” Adiabatic Lapse Rates Dry: 10°C/1000m Wet: approx 5°C/ 1000m Environmental: approx. 6.4°C/1000m Cloud Formation • When condensation occurs, latent heat stored in the water vapor is released, warming the parcel, which causes it to rise. • Due to the latent heat, it cools at a slower rate, the “wet” lapse rate. There is a good chance that it will cool less quickly than the atmosphere around it, and it will continue to rise, condensing as it goes. • This causes the cloud to have “vertical development”. Adiabatic Lapse Rates Dry: 10°C/1000m Wet: approx 5°C/ 1000m Environmental: approx. 6.4°C/1000m What makes air rise? • Warm air is less dense (lighter) than cool air. • Water vapor is less dense (lighter) than dry air. • Air near earth’s surface warms, picks up water vapor from near the surface of the Earth, then rises. • Adiabatic cooling lowers temperature to dew point, which causes cloud formation. • Air may be “helped” to rise by various means. Clouds Cloud families – based on height 1. Low: to 10,000 ft above the surface 2. Middle: 10,000-20,000 ft above the surface (prefix alto) 3. High – above 20,000 ft high (prefix cirro) 4. Clouds with vertical development Cloud classes - based on shape – stratiform – layered clouds – cumuliform - globular Cirrus – high altitude clouds • Formed from ice crystals, not liquid water drops. • Strong winds at altitude blow them into wisps (“mare’s tails”). • No rain (or snow) comes directly from them, but they are often an indication of unsettled weather in the near future. Altocumulus – Middle-layer Clouds “Mackerel Sky” Low Stratus Clouds • Horizontally layered, sheet-like clouds. • Form in relatively stable conditions when air stops rising as soon as condensation occurs. • Causes a gray overcast sky that may persist for days. • Nimbostratus clouds bring steady rain or snowfall (nimbo = rain). Cumulus clouds display vertical development • Fluffy, white clouds with flat bottoms and billowy tops that may rise as much as 4 miles up in the air. • Form from relatively unstable air which continues to rise after condensation takes place. Flat base of cloud is where condensation started. • Rain or snow comes in the form of brief, intense showers. Other Cumulus Clouds Fair weather cumulus Cumulonimbus Lenticular clouds form as moist air flows up and over a mountain peak. They are classified as altocumulus. Lenticular clouds indicate high winds aloft. Fog Fog is a cloud that forms at or very close to the ground. The picture shows an example of radiation fog, from air near the earth’s surface which cooled by radiation during the night. Fog usually disappears during the day, when the sun warms the earth and it begins to emit heat. San Diego Marine Layer The marine layer is an example of an advection fog: warm air over the ocean takes in water layer, but as prevailing west winds blow it over the land, the water vapor condenses Precipitation Precipitation • Clouds sometimes release moisture as precipitation – Occurs as rain, hail, snow or sleet – 3 mechanisms that cause air to rise, leading to cloud formation and precipitation • Orographic precipitation • Cyclonic precipitation • Convection-Convergence precipitation Orographic Precipitation • Prevailing winds push air up against, and then over, a mountain range • This process is responsible for our deserts in eastern San Diego County Rainfall Map • Orographic precipitation occurs on west side as air is lifted over mountains. • Great Central Valley has less rain than coastal regions. • Death Valley and Mojave Deserts are rainshadow deserts. Cyclonic Precipitation (Frontal Wedging) • When moving warm and cold air masses meet (a “front”), more dense cold air forces the less dense warm air to rise. • This happens when a storm (cyclone) passes through Convection-Convergence Precipitation • Forms when warm, moist air – – – – – Heats up near a hot surface Rises and cools adiabatically Condenses and forms clouds May form fair weather cumulus If conditions are unstable, can lead to precipitation in the form of thunderstorms. Convection/convergence Thunderstorms and Unstable Air Unstable air conditions caused by: • surface air that is very warm and moist • the environmental temperature lapse rate exceeds both dry and wet adiabatic rate (relatively cold, dry air aloft) – Rising air remains warmer than its surrounding air, causing strong, persistent convection (updrafts) – Clouds with intense vertical development – “towering cumulus” – Cumulonimbus clouds and thunderstorms Exploring Thunderstorms • Vertically developing clouds – Reach very high altitude – Are affected by high velocity winds – Become anvil-headed • Strong convective flows – Are created by precipitation dragging cool air down – And warm rising air taking its place • Hail and lightning are destructive by-products of thunderstorms The force exerted by the atmosphere • Gas molecules in the atmosphere bounce randomly, creating force in all directions. • In addition, the force of gravity causes atmospheric gas molecules to concentrate at lower elevations. More force (in all directions) at lower elevations. Barometric Pressure - force exerted by the gas molecules in the atmosphere over a given area • English unit is pound per square inch (psi). At sea level, the atmosphere exerts about 14 pounds of force on a one by one inch area (slightly bigger than a postage stamp). • How many postage stamps fit on the top of your head? • How many pounds of force does the atmosphere exert on the top of your head? • Why don’t you have a big headache right now? Barometric pressure • At 5 km (about 3 miles) of elevation, there are only half the gas molecules above you that there are at sea level. • Now your headache is due to altitude sickness! Measuring barometric pressure • If you evacuate a tube (i.e. remove all the air) and put it in a dish of liquid, the liquid will fill the tube as the air pressure pushes on the liquid in the dish. • If you tried this with a dish of water, the water would rise up to about 33 feet in the tube! Measuring barometric pressure • Using mercury, a very heavy liquid, we find that at normal sea-level barometric pressure, the liquid in the tube rises to a height of 760 mm (or 29.92 inches). • This apparatus is the original form of the barometer, a device used for measuring barometric pressure. Measuring barometric pressure • What is a normal barometric pressure in the units reported by the TV news weatherman? • Hint: the units are not psi, and not millimeters Answer • In the U.S., Mr. TV Weatherman reports barometric pressure in inches, with normal ranging from 29-31 inches (listen for it tonight). • Overseas, Mr. TV weatherman probably announces the barometric pressure in Millibars (1013 millibars = normal sea level pressure) • Meteorologists routinely use millibars for scientific applications. Measuring barometric pressure – the modern way • Mercury barometers are dangerous and difficult to use. • Modern aneroid barometers use changes within a partially evacuated chamber to move the pointer to the correct value. This graph shows how the barometric pressure (x axis – in millibars) decreases as the elevation increases and the number of gas molecules above gets less and less. Acid Rain • Burning of coal and petroleum release sulfur and nitrogen oxide gases • In moist air, these gases form acids • Atmospheric acids dissolve in water droplets and fall as acid rain • The damage often occurs over 100 miles away • Acid rain damage in Ontario, Canada can be traced to coal-burning power plants along the Ohio River Valley Photochemical Smog • Incompletely burned gasoline reacts with nitrogen oxides and oxygen in the presence of sunlight to become ozone (O3). • Ozone reacts again with gasoline exhaust to become smog Ozone and smog • Irritates the lungs and aggravates asthma • Increases the liklihood of heart disease • A suspected carcinogen Ozone high and low • The ozone layer in the stratosphere is a good thing because it protects life on Earth from harmful UV rays • The Ozone in the troposphere is a bad thing because it damages hearts and lungs. Ozone formation in stratosphere Depletion of the ozone layer • Ozone – O3 forms in the stratosphere and absorbs UV energy, which can be harmful • Halons and Chlorofluorocarbons (CFCs), – are organic compounds that destroy ozone in the ozone layer Depletion of the ozone layer • Ozone hole – reported in 1985 and linked to CFCs in Antarctic ice clouds • Many nations of the world agreed to reduce or stop use of these CFCs and halons • Most industrial countries no longer produce CFCs. • Since banning CFCs, the hole may be decreasing